Life on earth depends on the chemical element carbon, which is present in every living thing. Carbon is so important; it forms the basis for two branches of chemistry - organic chemistry and biochemistry.
1. Carbon is the basis for organic chemistry____
2. Carbon is a nonmetal that can bond with itself and many other chemical elements, ____
3. Elemental carbon can take the form of one of the hardest substances (diamond) ______
4. Carbon is made in the interiors of stars, ______
5. Carbon compounds have limitless uses. In its elemental form, diamond is a gemstone and used for drilling/cutting; graphite is used in pencils, as a lubricant, and to protect against rust; ___________
6. Carbon has the highest melting/sublimation point of the elements. The melting point of diamond is ~3550°C, _________
7. Pure carbon exists free in nature _____________
8. The origin of the name 'carbon' comes from the Latin word carbo, for charcoal. _______
9. Pure carbon is considered non-toxic, _______
10. Carbon is the fourth most abundant element in the universe - _____
a ______ as it occurs in all living organisms.
b ______or one of the softest (graphite).
c ______ though it was not produced in the Big Bang.
d ______ and has been known since prehistoric time.
e ______ forming nearly ten million compounds.
f ___ hydrogen, helium, and oxygen are found in higher amounts, by mass.
g _ although inhalation of fine particles, such as soot, can damage lung tissue.
h ______ The German and French words for charcoal are similar.
i ______ while charcoal is used to remove toxins, tastes, and odors.
j ______ with the sublimation point of carbon around 3800°C.
Read the definitions of a functional group. Which do you think is the best? Explain why you think so.
a) A functional group is a portion of a molecule that is a recognizable/classified group of boundatoms. In organic chemistry it is very common to see molecules comprised mainly of a carbonbackbone with functional groups attached to the chain. The functional group gives the moleculeits properties, regardless of what molecule contains it; they are centers of chemical reactivity.
b) Functional groups are specific groups of atoms or bonds within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of.
What functional groups can you name? Read Text B and check your answers.
Text B. Functional groups.
The concept of functional groups is central in organic chemistry, both as a means to classify structures and for predicting properties. A functional group is a molecular module, and the reactivity of that functional group is assumed, within limits, to be the same in a variety of molecules. Functional groups can have decisive influence on the chemical and physical properties of organic compounds. Molecules are classified on the basis of their functional groups. Alcohols, for example, all have the subunit C-O-H. All alcohols tend to be somewhat hydrophilic, usually form esters, and usually can be converted to the corresponding halides. Most functional groups feature heteroatoms (atoms other than C and H). Organic compounds are classified according to functional groups, alcohols, carboxylic acids, amines, etc.
Aliphatic compounds.
The aliphatic hydrocarbons are subdivided into three groups of homologous series according to their state of saturation:
· paraffins, which are alkanes without any double or triple bonds,
· olefins or alkenes which contain one or more double bonds, i.e. di-olefins (dienes) or poly-olefins.
· alkynes, which have one or more triple bonds.
The rest of the group is classed according to the functional groups present. Such compounds can be "straight-chain", branched-chain or cyclic. The degree of branching affects characteristics, such as the octane number or cetane number in petroleum chemistry.
Both saturated (alicyclic) compounds and unsaturated compounds exist as cyclic derivatives. The most stable rings contain five or six carbon atoms, but large rings (macrocycles) and smaller rings are common. The smallest cycloalkane family is the three-membered cyclopropane ((CH2)3). Saturated cyclic compounds contain single bonds only, whereas aromatic rings have an alternating (or conjugated) double bond. Cycloalkanes do not contain multiple bonds, whereas the cycloalkenes and the cycloalkynes do.
Aromatic compounds.
Aromatic hydrocarbons contain conjugated double bonds. This means that every carbon atom in the ring is sp2 hybridized, allowing for added stability. The most important example is benzene, the structure of which was formulated by Kekulé who first proposed the delocalization or resonance principle for explaining its structure. For "conventional" cyclic compounds, aromaticity is conferred by the presence of 4n + 2 delocalized pi electrons, where n is an integer. Particular instability (antiaromaticity) is conferred by the presence of 4n conjugated pi electrons.
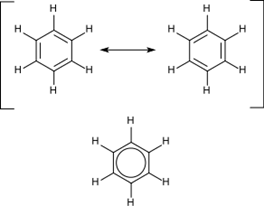
Benzene is one of the best-known aromatic compounds as it is one of the simplest and most stable aromatics.
Heterocyclic compounds.
The characteristics of the cyclic hydrocarbons are again altered if heteroatoms are present, which can exist as either substituents attached externally to the ring (exocyclic) or as a member of the ring itself (endocyclic). In the case of the latter, the ring is termed a heterocycle. Pyridine and furan are examples of aromatic heterocycles while piperidine and tetrahydrofuran are the corresponding alicyclic heterocycles. The heteroatom of heterocyclic molecules is generally oxygen, sulfur, or nitrogen, with the latter being particularly common in biochemical systems.
Heterocycles are commonly found in a wide range of products including aniline dyes and medicines. Additionally, they are prevalent in a wide range of biochemical compounds such as alkaloids, vitamins, steroids, and nucleic acids (e.g. DNA, RNA).
Rings can fuse with other rings on an edge to give polycyclic compounds. The purine nucleoside bases are notable polycyclic aromatic heterocycles. Rings can also fuse on a "corner" such that one atom (almost always carbon) has two bonds going to one ring and two to another. Such compounds are termed spiro and are important in a number of natural products.
Polymers.
One important property of carbon is that it readily forms chains, or networks, that are linked by carbon-carbon (carbon-to-carbon) bonds. The linking process is called polymerization, while the chains (or networks) are called polymers. The source compound is called a monomer.
There are two main groups of polymers: synthetic polymers and biopolymers. Synthetic polymers are artificially manufactured, and are commonly referred to as industrial polymers. Biopolymers occur within a respectfully natural environment, or without human intervention.
Common synthetic organic polymers are polyethylene (polythene), polypropylene, nylon, teflon (PTFE), polystyrene, polyesters, polymethylmethacrylate (called perspex and plexiglas), and polyvinylchloride (PVC).Both synthetic and natural rubber is a polymer.
Varieties of each synthetic polymer product may exist for purposes of a specific use. Changing the conditions of polymerization alters the chemical composition of the product and its properties. These alterations include the chain length, or branching, or the tacticity.
With a single monomer as a start, the product is a homopolymer. Secondary component(s) may be added to create a heteropolymer (co-polymer) and the degree of clustering of the different components can also be controlled. Physical characteristics, such as hardness, density, mechanical or tensile strength, abrasion resistance, heat resistance, transparency, color, etc. will depend on the final composition.
Biomolecules.
Biomolecular chemistry is a major category within organic chemistry which is frequently studied by biochemists. Many complex multi-functional group molecules are important in living organisms. Some are long-chain biopolymers, and these include peptides, DNA, RNA and the polysaccharides such as starches in animals and celluloses in plants. The other main classes are amino acids (monomer building blocks of peptides and proteins), carbohydrates (which includes the polysaccharides), the nucleic acids (which include DNA and RNA as polymers), and the lipids. In addition, animal biochemistry contains many small molecule intermediates which assist in energy production through the Krebs cycle (the citric acid cycle), and produces isoprene, the most common hydrocarbon in animals. Isoprenes in animals form the important steroid structural (cholesterol) and steroid hormone compounds; and in plants form terpenes, terpenoids, some alkaloids, and a class of hydrocarbons called biopolymer polyisoprenoids present in the latex of various species of plants, which is the basis for making rubber.
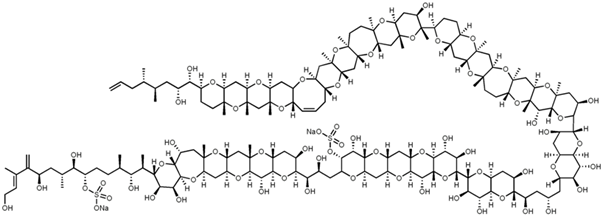
Maitotoxin is a complex organic biological toxin.
Small molecules.
In pharmacology, an important group of organic compounds is small molecules, also referred to as 'small organic compounds'. In this context, a small molecule is a small organic compound that is biologically active, but is not apolymer. In practice, small molecules have a molar mass less than approximately 1000 g/mol.
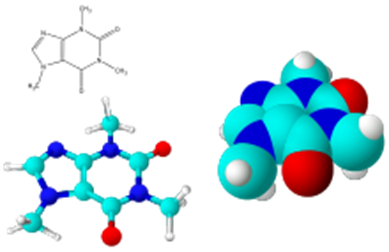
Molecular models of caffeine.
Fullerenes.
Fullerenes and carbon nanotubes, carbon compounds with spheroidal and tubular structures, have stimulated much research into the related field of materials science.
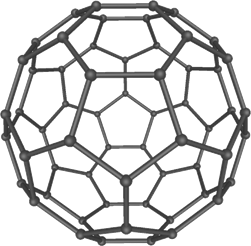
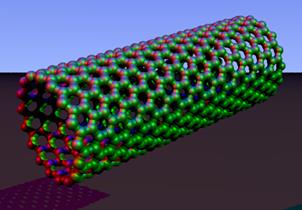
Buckminsterfullerene C60 (left) and carbon nanotubes (right) are two examples of structures in the fullerene family.
The first fullerene was discovered in 1985 by Sir Harold W. Kroto of the United Kingdom and by Richard E. Smalley and Robert F. Curl, Jr., of the United States. Using a laser to vaporize graphite rods in an atmosphere of helium gas, these chemists and their assistants obtained cagelike molecules composed of 60 carbon atoms (C60) joined together by single and double bonds to form a hollow sphere with 12 pentagonal and 20 hexagonal faces - a design that resembles a soccer ball. In 1996 the trio was awarded the Nobel Prize for their pioneering efforts. The C60 molecule was named buckminsterfullerene (or, more simply, the buckyball) after the American architect R. Buckminster Fuller, whose geodesic dome is constructed on the same structural principles.
Others.
Organic compounds containing bonds of carbon to nitrogen, oxygen and the halogens are not normally grouped separately. Others are sometimes put into major groups within organic chemistry and discussed under titles such as organosulfur chemistry, organometallic chemistry, organophosphorus chemistry and organosilicon chemistry.
6. Find the English equivalents of these words and phrases in the text:
Алифатические соединения; ароматические соединения; гетероциклические соединения; гидрофильный; гомологический ряд; дезоксирибонуклеиновая кислота; замещающий атом; малые молекулы; молярная масса; насыщение; несольватированный электрон; плотность; полициклическое соединение, кольца которого имеют один общий атом; предел прочности; разветвление; симметричность молекулярной структуры; сложные эфиры; сополимер; сопряжённые двойные связи; тройная связь; углеводы; углеродные нанотрубки; цетановое число; цикл трикарбоновых кислот.
7.Read these statements about Text B and decide if the they are true (T) or false (F) supporting your answers with necessary information from the text:
1. The concept of functional groups is central in inorganic chemistry, both as a means to classify structures and for predicting properties.
2. Any functional group can be regarded as a molecular module, and the reactivity of a functional group will always be the same in a variety of molecules.
3. A heteroatom is any atom that is not carbon or hydrogen.
4. The aliphatic hydrocarbons are subdivided into four groups of homologous series according to their state of saturation.
5. Cycloalkenes and cycloalkanes contain multiple bonds.
6. Kekulé was the first to propose the delocalization or resonance principle for explaining the structure of benzene.
7. Endocyclic heteroatoms are substituents attached externally to the ring.
8. Heterocyclic compounds are most abundant in such biochemical compounds as alkaloids, steroids and nucleic acids.
9. Biopolymers can be artificially manufactured but sometimes they occur within natural environment.
10. The abbreviation PVC stands for polymethylmethacrylate.
11. Polysaccharides belong to the class of amino acids.
12. A small molecule is a small organic compound that is biologically active, but is not a polymer. It has a molar mass less than 500 g/mol.
13. Carbon nanotubes are carbon compounds with spheroidal and tubular structures.
14. The C60 molecule called buckminsterfullerene is composed of 60 carbon atoms joined together by single and double bonds to form a hollow sphere with 12 pentagonal and 20 hexagonal faces.
15. Organic compounds containing bonds of carbon to nitrogen, oxygen and the halogens are grouped separately.