ENGLISH FOR CHEMI С AL INDUSTRY
Учебное пособие

Волгоград
2017
ББК Ш 143.21-92
Рецензенты:
кафедра «Английская филология» ВГСПУ,
зав. кафедрой, д-р филол. наук, профессор В. И. Карасик;
канд. пед. наук, доцент кафедры
иностранных языков ВГАФК И. В. Бганцева
Печатается по решению редакционно-издательского совета
Волгоградского государственного технического университета
Топоркова, О. В.
English for Chemical Industry: учебное пособие по развитию профессиональной языковой компетенции в области технологий химических процессов для студентов химико-технологического факультета. Английский язык / О. В. Топоркова, А. В. Герасимова, Т. Н. Синенко, Н. В. Стрепетова; под общей ред. О. В. Топорковой; ВолгГТУ. – Волгоград, 2017. – 128 с.
ISBN
Цель пособия – формирование компетенций ведения профессиональной коммуникации в области технологий химических процессов, развитие навыков делового общения в сфере химических технологий, а также совершенствование навыков самостоятельного чтения оригинальной англоязычной литературы по специальности.
В пособии представлены современные аутентичные тексты, отобран соответствующий языковой и речевой материал, разработана система продуктивных упражнений для закрепления лексики и грамматики и развития навыков чтения и устной речи по темам, связанным с проблемами химических процессов.
Учебное пособие предназначено для студентов химико-технологического факультета, слушателей специальных курсов и специалистов данного профиля, совершенствующих знания английского языка и профессиональные компетенции, а также навыкиобщения и чтения аутентичных текстов на английском языке.
Библиография: 27 назв.
ISBN
© Волгоградский государственный
технический университет, 2017
© О.В. Топоркова, А.В. Герасимова,
Т.Н. Синенко, Н.В. Стрепетова, 2017
ПРЕДИСЛОВИЕ
Учебное пособие «English for Chemical Industry» предназначено для студентов технических вузов, получающих образование по направлениям подготовки бакалавриата «Химическая технология», «Энерго- и ресурсосберегающие процессы в химической технологии, нефтехимии и биотехнологии». Данное учебное пособие предназначено, прежде всего, для студентов 2 курса и рассчитано на один год обучения.
Целью учебного пособия является развитие профессиональной языковой компетенции студентов, а именно: введение и закрепление в устной и письменной речи студентов лексического минимума по специальности, развитие навыков работы с текстами по специальности, совершенствование лексико-грамматических навыков, формирование у будущих специалистов навыков англоязычной профессиональной коммуникации. Пособие состоит из 2 разделов.
Первый раздел состоит из 8 подразделов (Units). Каждый юнит посвящен определенной теме (“What is chemistry?”, “Laboratory”, “Periodic Table”, “Matter”, “Inorganic chemistry”, “Organic chemistry”, “Plastics” “Environmental chemistry”), на усвоение которой нацелена система текстов и лексико-грамматических упражнений. Структура каждого подраздела включает: вводные задания, целью которых является знакомство с темой урока, лексический минимум (терминологию) по специальности, ряд аутентичных текстов по специальности, упражнения на словообразование закрепляемых в данном уроке терминологических единиц, лексические и грамматические упражнения, упражнения на совершенствование навыков работы с текстом по специальности, упражнения на развитие коммуникативной компетенции студентов. Все упражнения носят профессионально-ориентированный характер, учебный материал изложен согласно принципу «от простого к сложному».
Второй раздел учебного пособия (Grammar revision) предназначен для повторения грамматических тем, представляющих сложность для изучающих английский язык для специальных целей. Материал для упражнений взят из оригинальной английской и американской общенаучной и специальной литературы. Как правило, предложения, данные в упражнениях для перевода, приведены согласно степени сложности. В ряде случаев упражнения по наиболее трудным для усвоения темам предваряет краткий комментарий повторяемого грамматического явления.
Разнообразные формы работы – в парах, малых группах, ситуации ролевого общения – стимулируют творческую активность и поддерживают интерес к общению на английском языке.
Учебное пособие «English for Chemical Industry» написано в соответствии с требованиями Федерального государственного образовательного стандарта Высшего образования при реализации основных образовательных программ бакалавриата технических направлений подготовки по дисциплине «Иностранный язык (английский)».
UNIT I. WHAT IS CHEMISTRY?
1. Discuss the following questions:
1 How would you define chemistry?
2. What comes to your mind when you hear the word “chemistry”?
2. You are going to read the text about chemistry. Study the vocabulary from the text:
| 1. absorption
| поглощение
|
| 2. color
| цвет
|
| 3. composition
| состав
|
| 4. compound
| соединение
|
| 5.determination
| определение
|
| 6. drug
| лекарствo
|
| 7. explosive
| взрывчатое веществo
|
| 8. heat
| тепло, нагревать
|
| 9. insecticide
| средство от насекомых, инсектицид
|
| 10. light
| свет, легкий
|
| 11. matter
| материя, вещество
|
| 12. mixture
| смесь
|
| 13. paint
| краска
|
| 14. perfume
| духи, аромат
|
| 15. property
| свойство
|
| 16. smell
| запах
|
| 17. soap
| мыло
|
| 18. solubility
| растворимость
|
| 19. substance
| вещество, материя
|
3. Read Text A and answer the following questions and say what chemistry is and what chemistry studies:
UNIT II. LABORATORY.
1. Discuss the following questions:
1. Is a chemical laboratory a dangerous place? Why?
2. What should you know before you go to a laboratory?
Speaking. Remember any accident that happened to you or your fellow student in the lab. What happened? What were you/he/she doing when it happened? Did you/he/she get injured? What rules did you/he/she break? What should you/he/she have done to prevent it? Tell the group about it
UNIT III. PERIODIC TABLE
1. Discuss the following questions:
1. Who established the Periodic System of Elements? What is the popular legend connected with its establishment?
2. Why is the Periodic table so important for studying chemistry?
UNIT IV. MATTER.
1. Discuss the following questions:
1. What is matter?
2. What states of matter can you name?
Text A. States of matter.
There are four main states of matter: solids, liquids, gases and plasmas. Each of these states is also known as a phase. Elements and compounds can move from one phase to another phase when special physical forces are present. One example of those forces is temperature. The phase or state of matter can change when the temperature changes. Generally, as the temperature rises, matter moves to a more active state.
Phase describes a physical state of matter. The key word to notice is physical. Things only move from one phase to another by physical means. If energy is added (like increasing the temperature or increasing pressure) or if energy is taken away (like freezing something or decreasing pressure) you have created a physical change.
One compound or element can move from phase to phase, but still be the same substance. You can see water vapor over a boiling pot of water. That vapor (or gas) can condense and become a drop of water. If you put that drop in the freezer, it would become a solid. No matter what phase it was in, it was always water. It always had the same chemical properties. On the other hand, a chemical change would change the way the water acted, eventually making it not water, but something completely new.
3. What is a ‘phase transition’? Read Text B and fill in the gaps with the following terms:
a) ionization
b) sublimation
c) freezing
d) deionization
e) melting
f) vaporization
g) deposition
h) condensation
Acids.
Acids are compounds that produce H+ ions when dissolved in water. Examples of acids include sulfuric acid (H2SO4), hydrochloric acid (HCl), hydrofluoric acid (HF), nitric acid (HNO3) and phosphoric acid (H3PO4). Most acids can be dissolved in water and are corrosive, and those that can be ingested have a sour taste. In water, HCl is decomposed in H+ and Cl-
HCl → (H+) + (Cl-)
Bases.
Bases are compounds that produce OH- (hydroxyl ions) when dissolved in water. They are usually found in household products. Some common bases are ammonia hydroxide (NH4OH), potassium hydroxide (KOH), calcium hydroxide or caustic lime Ca(OH)2, and sodium hydroxide or caustic soda (NaOH). In water, KOH dissociates in K+ and OH-:
KOH → (K+) + (OH-)
Salts.
Salts are compounds that result from the reaction between an acid and a base. They are ionic compounds formed by two oppositely charged ions (atoms that are not electrically neutral because they have lost or gained one or more electrons). For example, table salt or sodium chloride (NaCl) is formed by the bonding an anion (positively charged ion) and a cation (negatively charged ion): Na+ and Cl-.
Some common salts include sodium chloride or table salt (NaCl), calcium chloride (CaCl2), magnesium chloride (MgCl2), and potassium chloride (KCl). Most salts can be dissolved in water to form a solution of the ions. Ions derived from salts like Na+, Mg+2, and K+ are critical for the functioning of the human body. In water, CaCl2 is decomposed in the following way:
CaCl2 → (Ca+2) + (Cl-)
Oxides.
Oxides are compounds that contain at least one oxygen atom combined with another element. Oxygen is usually in the form of an anion (O2-). Transition metal oxides such as titanium (III) oxide (Ti2O3) and iron (III) oxide (Fe2O3) have useful magnetic and catalytic properties.
2. Comprehension check. Read these statements and decide if they are true (T), false (F) or the information is not stated in the text (NS) supporting your answers with necessary information from Text A:
1. Carbon is the most prevalent element in the universe.
2. Inorganic chemistry is the study of the formation and properties of compounds that contain carbon-hydrogen bonds.
3. Some organic compounds can be used in the production of household products such as washing powder or different detergents.
4. Sodium oxide is used as table salt.
5. Inorganic compounds are represented by acids, oxides, and salts.
6. Acids are compounds that produce H+ ions when dissolved in water.
7. Most acids can be dissolved in water and are corrosive, and those that can be ingested have a bitter taste.
8. Bases are compounds that produce hydroxyl ions when dissolved in water.
9. Salts are inorganic compounds formed by anions and cations.
10. Most salts can be dissolved in alkaline solutions.
11. Oxides are compounds that contain one or more atoms of oxygen combined with another element. Oxygen is usually in the form of a cation.
I. Combination Reactions
Two or more reactants form one product in a combination reaction. An example of a combination reaction is the formation of sulfur dioxide when sulfur is burned in air:
S (s) + O2 (g) → SO2 (g)
II. Decomposition Reactions
In a decomposition reaction, a compound breaks down into two or more substances. Decomposition usually results from electrolysis or heating. An example of a decomposition reaction is the breakdown of mercury (II) oxide into its component elements.
2HgO (s) + heat → 2Hg (l) + O2 (g)
Binary compounds
Acids
I. Hydrogen acids
HCl - hydrochlor ic acid
HF - _______________
II. Oxoacids/Oxyacids
H2SO4 - sulfur ic acid
H2SO3 - sulfur ous acid
HNO3 - _______________
HNO2 - _______________
UNIT VI. ORGANIC CHEMISTRY.
Text B. Functional groups.
The concept of functional groups is central in organic chemistry, both as a means to classify structures and for predicting properties. A functional group is a molecular module, and the reactivity of that functional group is assumed, within limits, to be the same in a variety of molecules. Functional groups can have decisive influence on the chemical and physical properties of organic compounds. Molecules are classified on the basis of their functional groups. Alcohols, for example, all have the subunit C-O-H. All alcohols tend to be somewhat hydrophilic, usually form esters, and usually can be converted to the corresponding halides. Most functional groups feature heteroatoms (atoms other than C and H). Organic compounds are classified according to functional groups, alcohols, carboxylic acids, amines, etc.
Aliphatic compounds.
The aliphatic hydrocarbons are subdivided into three groups of homologous series according to their state of saturation:
· paraffins, which are alkanes without any double or triple bonds,
· olefins or alkenes which contain one or more double bonds, i.e. di-olefins (dienes) or poly-olefins.
· alkynes, which have one or more triple bonds.
The rest of the group is classed according to the functional groups present. Such compounds can be "straight-chain", branched-chain or cyclic. The degree of branching affects characteristics, such as the octane number or cetane number in petroleum chemistry.
Both saturated (alicyclic) compounds and unsaturated compounds exist as cyclic derivatives. The most stable rings contain five or six carbon atoms, but large rings (macrocycles) and smaller rings are common. The smallest cycloalkane family is the three-membered cyclopropane ((CH2)3). Saturated cyclic compounds contain single bonds only, whereas aromatic rings have an alternating (or conjugated) double bond. Cycloalkanes do not contain multiple bonds, whereas the cycloalkenes and the cycloalkynes do.
Aromatic compounds.
Aromatic hydrocarbons contain conjugated double bonds. This means that every carbon atom in the ring is sp2 hybridized, allowing for added stability. The most important example is benzene, the structure of which was formulated by Kekulé who first proposed the delocalization or resonance principle for explaining its structure. For "conventional" cyclic compounds, aromaticity is conferred by the presence of 4n + 2 delocalized pi electrons, where n is an integer. Particular instability (antiaromaticity) is conferred by the presence of 4n conjugated pi electrons.
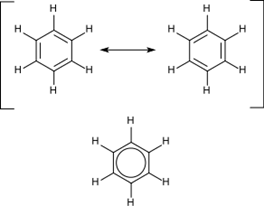
Benzene is one of the best-known aromatic compounds as it is one of the simplest and most stable aromatics.
Heterocyclic compounds.
The characteristics of the cyclic hydrocarbons are again altered if heteroatoms are present, which can exist as either substituents attached externally to the ring (exocyclic) or as a member of the ring itself (endocyclic). In the case of the latter, the ring is termed a heterocycle. Pyridine and furan are examples of aromatic heterocycles while piperidine and tetrahydrofuran are the corresponding alicyclic heterocycles. The heteroatom of heterocyclic molecules is generally oxygen, sulfur, or nitrogen, with the latter being particularly common in biochemical systems.
Heterocycles are commonly found in a wide range of products including aniline dyes and medicines. Additionally, they are prevalent in a wide range of biochemical compounds such as alkaloids, vitamins, steroids, and nucleic acids (e.g. DNA, RNA).
Rings can fuse with other rings on an edge to give polycyclic compounds. The purine nucleoside bases are notable polycyclic aromatic heterocycles. Rings can also fuse on a "corner" such that one atom (almost always carbon) has two bonds going to one ring and two to another. Such compounds are termed spiro and are important in a number of natural products.
Polymers.
One important property of carbon is that it readily forms chains, or networks, that are linked by carbon-carbon (carbon-to-carbon) bonds. The linking process is called polymerization, while the chains (or networks) are called polymers. The source compound is called a monomer.
There are two main groups of polymers: synthetic polymers and biopolymers. Synthetic polymers are artificially manufactured, and are commonly referred to as industrial polymers. Biopolymers occur within a respectfully natural environment, or without human intervention.
Common synthetic organic polymers are polyethylene (polythene), polypropylene, nylon, teflon (PTFE), polystyrene, polyesters, polymethylmethacrylate (called perspex and plexiglas), and polyvinylchloride (PVC).Both synthetic and natural rubber is a polymer.
Varieties of each synthetic polymer product may exist for purposes of a specific use. Changing the conditions of polymerization alters the chemical composition of the product and its properties. These alterations include the chain length, or branching, or the tacticity.
With a single monomer as a start, the product is a homopolymer. Secondary component(s) may be added to create a heteropolymer (co-polymer) and the degree of clustering of the different components can also be controlled. Physical characteristics, such as hardness, density, mechanical or tensile strength, abrasion resistance, heat resistance, transparency, color, etc. will depend on the final composition.
Biomolecules.
Biomolecular chemistry is a major category within organic chemistry which is frequently studied by biochemists. Many complex multi-functional group molecules are important in living organisms. Some are long-chain biopolymers, and these include peptides, DNA, RNA and the polysaccharides such as starches in animals and celluloses in plants. The other main classes are amino acids (monomer building blocks of peptides and proteins), carbohydrates (which includes the polysaccharides), the nucleic acids (which include DNA and RNA as polymers), and the lipids. In addition, animal biochemistry contains many small molecule intermediates which assist in energy production through the Krebs cycle (the citric acid cycle), and produces isoprene, the most common hydrocarbon in animals. Isoprenes in animals form the important steroid structural (cholesterol) and steroid hormone compounds; and in plants form terpenes, terpenoids, some alkaloids, and a class of hydrocarbons called biopolymer polyisoprenoids present in the latex of various species of plants, which is the basis for making rubber.
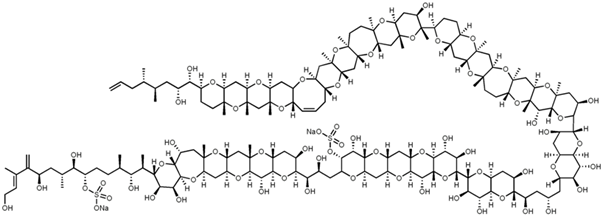
Maitotoxin is a complex organic biological toxin.
Small molecules.
In pharmacology, an important group of organic compounds is small molecules, also referred to as 'small organic compounds'. In this context, a small molecule is a small organic compound that is biologically active, but is not apolymer. In practice, small molecules have a molar mass less than approximately 1000 g/mol.
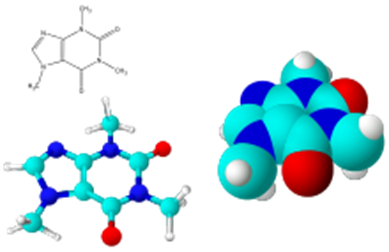
Molecular models of caffeine.
Fullerenes.
Fullerenes and carbon nanotubes, carbon compounds with spheroidal and tubular structures, have stimulated much research into the related field of materials science.
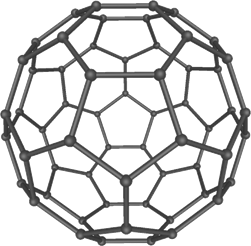
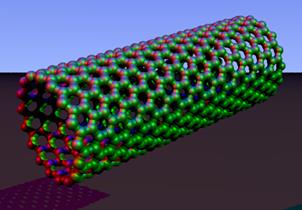
Buckminsterfullerene C60 (left) and carbon nanotubes (right) are two examples of structures in the fullerene family.
The first fullerene was discovered in 1985 by Sir Harold W. Kroto of the United Kingdom and by Richard E. Smalley and Robert F. Curl, Jr., of the United States. Using a laser to vaporize graphite rods in an atmosphere of helium gas, these chemists and their assistants obtained cagelike molecules composed of 60 carbon atoms (C60) joined together by single and double bonds to form a hollow sphere with 12 pentagonal and 20 hexagonal faces - a design that resembles a soccer ball. In 1996 the trio was awarded the Nobel Prize for their pioneering efforts. The C60 molecule was named buckminsterfullerene (or, more simply, the buckyball) after the American architect R. Buckminster Fuller, whose geodesic dome is constructed on the same structural principles.
Others.
Organic compounds containing bonds of carbon to nitrogen, oxygen and the halogens are not normally grouped separately. Others are sometimes put into major groups within organic chemistry and discussed under titles such as organosulfur chemistry, organometallic chemistry, organophosphorus chemistry and organosilicon chemistry.
6. Find the English equivalents of these words and phrases in the text:
Алифатические соединения; ароматические соединения; гетероциклические соединения; гидрофильный; гомологический ряд; дезоксирибонуклеиновая кислота; замещающий атом; малые молекулы; молярная масса; насыщение; несольватированный электрон; плотность; полициклическое соединение, кольца которого имеют один общий атом; предел прочности; разветвление; симметричность молекулярной структуры; сложные эфиры; сополимер; сопряжённые двойные связи; тройная связь; углеводы; углеродные нанотрубки; цетановое число; цикл трикарбоновых кислот.
7.Read these statements about Text B and decide if the they are true (T) or false (F) supporting your answers with necessary information from the text:
1. The concept of functional groups is central in inorganic chemistry, both as a means to classify structures and for predicting properties.
2. Any functional group can be regarded as a molecular module, and the reactivity of a functional group will always be the same in a variety of molecules.
3. A heteroatom is any atom that is not carbon or hydrogen.
4. The aliphatic hydrocarbons are subdivided into four groups of homologous series according to their state of saturation.
5. Cycloalkenes and cycloalkanes contain multiple bonds.
6. Kekulé was the first to propose the delocalization or resonance principle for explaining the structure of benzene.
7. Endocyclic heteroatoms are substituents attached externally to the ring.
8. Heterocyclic compounds are most abundant in such biochemical compounds as alkaloids, steroids and nucleic acids.
9. Biopolymers can be artificially manufactured but sometimes they occur within natural environment.
10. The abbreviation PVC stands for polymethylmethacrylate.
11. Polysaccharides belong to the class of amino acids.
12. A small molecule is a small organic compound that is biologically active, but is not a polymer. It has a molar mass less than 500 g/mol.
13. Carbon nanotubes are carbon compounds with spheroidal and tubular structures.
14. The C60 molecule called buckminsterfullerene is composed of 60 carbon atoms joined together by single and double bonds to form a hollow sphere with 12 pentagonal and 20 hexagonal faces.
15. Organic compounds containing bonds of carbon to nitrogen, oxygen and the halogens are grouped separately.
Speaking.
Organic chemists at all levels are generally employed by pharmaceutical, biotech, chemical, consumer product, and petroleum industries. Chemists in industry mainly work in development, while chemists in academia are involved in more basic research. The federal and local governments also hire organic chemists (e.g., Federal Service for Supervision of Healthcare, Federal Medical-Biological Agency or Federal Service for Veterinary and Phytosanitary Supervision). Work in groups of 3 or 4 and discuss which industry you would like to work at. Consider advantages and drawbacks of working at each industry and choose the one that fits you best.
UNIT VII. PLASTICS.
1. Discuss the following questions:
1. Have a look at the desk you are sitting at. What plastic things can you name?
2. What is the basic chemical element in plastics formula?
2. You are going to read Text A about polymers. Study new vocabulary from the text:
| 1. branch
| разветвленный
|
| 2. cellulose
| 1) клетчатка; 2) целлюлоза
|
| 3. chain
| цепь
|
| 4. chemicals
| хим. вещества
|
| 5. coil
| 1) сердечник; 2) спираль
|
| 6. fibre
| волокно, нить
|
| 7. flexible
| гибкий
|
| 8. insulator
| 1) непроводник; 2) изоляционный материал
|
| 9. resin
| смола
|
| 10. rubber
| резина, каучук
|
| 11. thermosetting plastics
| термореактивные пластмассы
|
| 12. to decompose
| разлагаться
|
| 13. to harden
| делать твердым
|
| 14. to prevent
| предотвращать
|
| 15. to soften
| смягчать
|
| 16. to subject
| подвергать
|
| 17. transparent
| прозрачный
|
3. Look through Text A and fill in the word-web:

Text A. Plastics.
Plastics are non-metallic, synthetic, carbon-based materials. They can be moulded, shaped, or extruded into flexible sheets, films, or fibres. Plastics are synthetic polymers. Polymers consist of long-chain molecules made of large numbers of identical small molecules (monomers). The chemical nature of a plastic is defined by the monomer (repeating unit) that makes up the chain of the polymer. Polyethene is a polyolefin; its monomer unit is ethene (formerly called ethylene). Other categories are acrylics (such as polymethylmethacrylate), styrenes (such as polystyrene), vinys (such as polyvinyl chloride (PVC)), polyesters, polyurethanes, polyamides (such as nylons), polyethers, acetals, phenolics, cellulosics, and amino resins. The molecules can be either natural — like cellulose, wax, and natural rubber — or synthetic — in polyethene and nylon. In co-polymers, more than one monomer is used.
The giant molecules of which polymers consist may be linear, branched, or cross-linked, depending on the plastic. Linear and branched molecules are thermoplastic (soften when heated), whereas cross-linked molecules are thermosetting (harden when heated).
Most plastics are synthesized from organic chemicals or from natural gas or coal. Plastics are light-weight compared to metals and are good electrical insulators. The best insulators now are epoxy resins and teflon. Teflon or polytetrafluoroethene (PTFE) was first made in 1938 and was produced commercially in 1950.
Plastics can be classified into several broad types.
1. Thermoplastics soften on heating, then harden again when cooled. Thermoplastic molecules are also coiled and because of this they are flexible and easily stretched.
Typical example of thermoplastics is polystyrene. Polystyrene resins are characterized by high resistance to chemical and mechanical stresses at low temperatures and by very low absorption of water. These properties make the polystyrenes especially suitable for radio-frequency insulation and for parts used at low temperatures in refrigerators and in airplanes. PET (polyethene terephthalate) is a transparent thermoplastic used for soft-drinks bottles. Thermoplastics are also viscoelastic, that is, they flow (creep) under stress. Examples are polythene, polystyrene and PVC.
2. Thermosetting plastics (thermosets) do not soften when heated, and with strong heating they decompose. In most thermosets final cross-linking, which fixes the molecules, takes place after the plastic has already been formed.
Thermosetting plastics have a higher density than thermoplastics. They are less flexible, more difficult to stretch, and are less subjected to creep. Examples of thermosetting plastics include urea-formaldehyde or polyurethane and epoxy resins, most polyesters, and phenolic polymers such as phenol-formaldehyde resin.
3. Elastomers are similar to thermoplastics but have sufficient cross-linking between molecules to prevent stretching and creep.
4. Read and translate the following words from English into Russian:
To compose – composed – composing - composition; to decompose – decomposed – decomposing – decomposite - decomposition; to harden – hardener; to prevent – preventer – prevention - preventative; to soften - softening; to absorb – absorber – absorbing – absorbent – absorption – absorptive - absorptivity
5. Find English equivalents in the text:
Cинтетические полимеры, молекулы с длинными цепями, характерные свойства полимера, синтезируются из органических химических веществ, хороший электрический изолятор, размягчаться при нагревании, затвердевать при охлаждении, гибкий и легко растяжимый, течь под нагрузкой, более высокая плотность, менее подвержены ползучести, достаточная взаимосвязь между молекулами
6. Comprehension check. Read Text A and answer the following questions:
1. What is the definition of plastics?
2. What do polymers consist of?
3. What are long-chain molecules made of?
4. What are the main types of polymers?
5. Give examples of plastics belonging to these types.
6. What plastics are the best electrical insulators?
7. Describe the difference between thermoplastics and thermosets.
8. What are the main types of structures of polymers?
9. What are the most important properties of plastics?
10. Give the examples of various uses of plastics because of their characteristic properties.
7. Fill in the correct word:
Composition, decomposes, absorbent, prevented, decomposed, absorb, preventative, absorption.
1. If bodies radiate more energy than they ____ from surrounding bodies they will cool. 2. Sodium hydroxide solution is the best _____ for carbon dioxide. 3. The process of absorbing is called ______. 4. Zinc carbonate when heated ______ into zinc oxide and carbon dioxide. 5. Unless we get more funding we’ll be ______ from finishing our experimental programme. 6. This scientist took all possible ______ measures in his laboratory. 7. Water can be ______ into hydrogen and oxygen. 8. A chemical composition of this new polymer is unique.
8. Translate into English using the target vocabulary:
1. Длинные цепи молекул полимеров состоят из одинаковых небольших молекул мономеров. 2. Сополимеры состоят из двух и более мономеров. 3. Пластмассы можно получать в виде листов, тонких пленок, волокон или гранул. 4. Молекулы полимеров могут быть линейными, ветвящимися или с поперечными связями. 5. Малый вес пластмасс и хорошие электроизоляционные свойства позволяют использовать их в радиоэлектронике и электроприборах, а также вместо металлов. 6. Молекулы термопластов имеют извитую форму и, поэтому, они гибкие и легко растяжимы. 7. Эластомеры имеют большое число поперечных связей между молекулами.
9. Open the brackets:
The packaging industry 1) (to be) a leading user of plastics. High-density polyethene (HPDE) 2) (to use) for some thicker plastic films, such as those used for plastic waste bags and containers. Other packaging plastics 3) (to include) polypropene, polystyrene, polyvinyl chloride (PVC), and polyvinylidene chloride. Polyvinylidene chloride 4) (to use) primarily for its barrier properties, which can keep gases such as oxygen from passing into or out of a package. Similarly, polypropene 5) (to be) an effective barrier against water vapour and as a fibre for carpeting and rope.
The building industry 6) (to be) a major consumer of plastics, including many of the packaging plastics mentioned above. PVC also 7) (to use) in sheets for building materials and similar items. Many plastics 8) (to use) to insulate cables and wires, and polystyrene in the form of foam serves as insulation for walls, roofs, and other areas.
Many other industries, especially motor manufacturing, also 9) (to depend) on plastics. Tough engineering plastics 10) (to find) in vehicle components like fuel lines, fuel pumps, and electronic devices. Plastics are also used for interior panelling, seats, and trim. Many car bodies 11) (to make) of fibreglass-reinforced plastic.
10. Speaking. Tell your groupmates about:
1) inventor(s) of plastics;
2) about modern scientists working on improving properties of plastics.
11. You are going to read Text B. Study new vocabulary from the text:
| 1. adhesion
| a. прилипание
|
| 2. adhesive
| b. клей
|
| 3. bond
| c. связь
|
| 4. capacitor
| d. эл. конденсатор
|
| 5. casting
| e. литье
|
| 6. catalyst
| f. катализатор
|
| 7.coating
| g. слой, покрытие
|
| 8. durable
| h. прочный
|
| 9. envelope
| i. зд. обрамление
|
| 10. foam
| j. пена
|
| 11. granule
| k. гранула
|
| 12. impact
| l. удар
|
| 13. lattice
| m. латекс
|
| 14. modifier
| n. модификатор
|
| 15. paste
| o. паста
|
| 16. syringe
| p. шприц
|
| 17. void
| q. пустота
|
| 18. yield
| r. выход
|
12. Read the text “Types of plastics” and say what the following abbreviations stand for:
PVC; LDPE; HDPE
Text B. Types of plastics.
1. Epoxy resin.
Epoxy resin is a thermoset plastic containing epoxy groups. Epoxy resin hardens when it is mixed with solidifier and plasticizer. Plasticizers make a polymer more flexible.
Epoxy resins have outstanding adhesion, toughness, and resistance to attack from chemicals. They form strong bonds and have excellent electrical insulation properties. Large, complex, void-free castings can be made from them. They are also used as adhesives, and in composites for boat building and sports equipment.
2. PVC (polyvinyl chloride)
PVC (polyvinyl chloride) is a thermoplastic polymer made from vinyl chloride is a colourless solid with outstanding resistance to water, alcohols, and concentrated acids and alkalis. It is obtainable as granules, solutions, lattices, and pastes. When compounded with plasticizers, it yields a flexible material more durable than rubber. It is widely used for cable and wire insulation, in chemical plants, and in the manufacture of protective garments. Blow moulding of unplasticized PVC produces clear, tough bottles which do not affect the flavour of their contents. PVC is also used for production of tubes or pipes.
3. Polystyrene.
Polystyrene is a thermoplastic produced by the polymerization of styrene. The electrical insulating properties of polystyrene are outstandingly good and it is relatively unaffected by water. Typical applications include light fixtures, toys, bottles, lenses, capacitor dielectrics, medical syringes, and light-duty industrial components. Extruded sheets of polystyrene are widely used for packaging, envelope windows, and photographic film. Its resistance to impact can be improved by the addition of rubber modifiers. Polystyrene can be readily foamed; the resulting foamed polystyrene is used extensively for packaging.
4. Polythene (polyethene, polyethylene)
Polythene (polyethene, polyethylene) is a plastic made from ethane. It is one of the most widely used important thermoplastic polymers. It was first developed by the polymerization of ethane at a pressure of 2,000 bar at 200°C. This produced low-density polythene (LDPE). A relatively high-density form (HDPE) was synthesized in the 1950s using a complex catalyst. Polythene is a white waxy solid with very low density, reasonable strength and toughness, but low stiffness. It is easily moulded and has a wide range of uses in containers, packaging, pipes, coatings, and insulation.
Text C. Composite materials
The combinations of two or more different materials are called composite materials. They usually have unique mechanical and physical properties because they combine the best properties of different materials. For example, a fibre-glass reinforced plastic combines the high strength of thin glass fibres with the ductility and chemical resistance of plastic. Nowadays composites are being used for structures such as bridges, boat-building etc.
Composite materials usually consist of synthetic fibres within a matrix, a material that surrounds and is tightly bound to the fibres. The most widely used type of composite material is polymer matrix composites (PMCs). PMCs consist of fibres made of a ceramic material such as carbon or glass embedded in a plastic matrix. Usually the fibres make up about 60 per cent by volume. Composites with metal matrices or ceramic matrices are called metal matrix composites (MMCs) and ceramic matrix composites (CMCs), respectively.
Continuous-fibre composites are generally required for structural applications. The specific strength (strength-to-density ratio) and specific stiffness (elastic modulus-to-density ratio) of continuous carbon fibre PMCs, for example, can be better than metal alloys have. Composites can also have other attractive properties, such as high thermal or electrical conductivity and a low coefficient of thermal expansion.
Although composite materials have certain advantages over conventional materials, composites also have some disadvantages. For example, PMCs and other composite materials tend to be highly anisotropic — that is, their strength, stiffness, and other engineering properties are different depending on the orientation of the composite material. For example, if a PMC is fabricated so that all the fibres are lined up parallel to one another, then the PMC will be very stiff in the direction parallel to the fibres, but not stiff in the perpendicular direction. The designer who uses composite materials in structures subjected to multidirectional forces, must take these anisotropic properties into account. Also, forming strong connections between separate composite material components is difficult.
The advanced composites have high manufacturing costs. Fabricating composite materials is a complex process. However, new manufacturing techniques are developed. It will become possible to produce composite materials at higher volumes and at a lower cost than is now possible, accelerating the wider exploitation of these materials.
20. Study Text C and find the adequate English equivalents of the following words and phrases:
Композитные материалы, уникальные механические качества, керамический, полимерные матричные композиты, иметь некоторые недостатки, составлять 60% объема, углепластик, привлекательные качества; структура, подвергающаяся воздействию разнонаправленных сил, волокна параллельны, прочные связи, инженер-конструктор, свойства
21. Comprehension check. Answer the following questions:
1. What are the best properties of fibre-glass?
2. What do composite materials usually consist of?
3. What is used as a matrix in composites?
4. What is used as a filler or a fiber in composites?
5. How are the composite materials with ceramic and metal matrices called?
6. What are the advantages of composites?
7. What are the disadvantages of composites?
8. Why anisotropic properties of composites should be taken into account?
22. Translate into Russian:
1. PMC is fabricated so that all the fibres are lined up parallel to one another. 2. Forming strong connections between separate composite material components is difficult. 3. Fabricating composite materials is a complex process. 4. Composite materials have certain advantages over conventional materials. 5. Nowadays, composites are being used for structures such as bridges, boat-building etc. 6. Continuous-fibre composites are generally required for structural applications.
23. Fill in the correct word from the list:
Foams, an extruder, a polymer, composites, fibre, chains, plasticizers, bonds, manufacturing, additives, raw materials.
1. The first stage in ______ plastic is polymerization. 2. Chemical ______ are often used in plastics to produce some desired characteristic. 3. These oil-based ______ are relatively widely available and inexpensive. 4. ________ make a polymer more flexible, lubricants reduce problems with friction, and pigments add colour. 5. Many plastics are manufactured as _______. 6. Plastic _______ are composites of plastic. 7. _______ is a device that pumps a plastic through a desired die or shape. 8. Silicon does not form double _______ or long silicon _______. 9. Antioxidants protect ______ from chemical degradation by oxygen or ozone; similarly, ultraviolet stabilizers protect against weathering. 10. Polypropene is also often used in housewares and as _____ for carpeting and rope.
24. Read Text D and choose a title for each paragraph:
a. Additives
b. Raw Materials.
c. Shaping and Finishing.
d. Use.
e. Synthesizing the Polymer.
TEXT A. R/S STATEMENTS.
Risk and Safety Statements, also known as R/S numbers, R/S phrases or R/S sentences, is a system of hazard codes and phrases for labeling dangerous chemicals and compounds. The R/S statement of a compound consists of a risk part (R) and a safety part (S), each followed by a combination of numbers. Each number corresponds to a phrase.
In 2015, the risk and safety statements were replaced by hazard statements and precautionary statements in the course of harmonising classification, labeling and packaging of chemicals by introduction of the UN Globally Harmonized System of Classification and Labelling of Chemicals (GHS).
Example:
The R/S statement code for fuming hydrochloric acid (37%):
R: 34-37 S: 26-36-45.
The corresponding English language phrases:
Risks
R: 34 Causes burns
R: 37 Irritating to the respiratory system.
Safety
S: 26 In case of contact with eyes rinse immediately with plenty of water and seek medical advice.
S: 36 Wear suitable protective clothing.
S: 45 In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
Dashes separate the phrase numbers. They are not to be confused with range indicators.
Example: R: 34-37 Causes burns, irritating to the respiratory system.
Slashes indicate fixed combinations of single phrases.
Example: R: 36/37/38 Irritating to eyes, respiratory system and skin.
More examples of risk phrases:
| Code
| Phrase
|
| R1
| Explosive when dry
|
| R2
| Risk of explosion by shock, friction, fire or other sources of
ignition
|
| R4
| Forms very sensitive explosive metallic compounds
|
| R5
| Heating may cause an explosion
|
| R6
| Explosive with or without contact with air
|
| R7
| May cause fire
|
| R8
| Contact with combustible material may cause fire
|
| R12
| Extremely flammable
|
| R14
| Reacts violently with water
|
| R15
| Contact with water liberates extremely flammable gases
|
| R16
| Explosive when mixed with oxidising substances
|
| R18
| In use, may form flammable/explosive vapor-air mixture
|
| R19
| May form explosive peroxides
|
| R20
| Harmful by inhalation
|
| R21
| Harmful in contact with skin
|
| R22
| Harmful if swallowed
|
| R35
| Causes severe burns
|
| R37
| Irritating to respiratory system
|
| R38
| Irritating to skin
|
| R39
| Danger of very serious irreversible effects
|
| R40
| Limited evidence of a carcinogenic effect
|
| R44
| Risk of explosion if heated under confinement
|
| R46
| May cause inheritable genetic damage
|
| R48
| Danger of serious damage to health by prolonged exposure
|
| R49
| May cause cancer by inhalation
|
| R53
| May cause long-term adverse effects in the aquatic environment
|
| R54
| Toxic to flora
|
| R55
| Toxic to fauna
|
| R56
| Toxic to soil organisms
|
| R57
| Toxic to bees
|
| R58
| May cause long-term adverse effects in the environment
|
| R59
| Dangerous for the ozone layer
|
| R67
| Vapours may cause drowsiness and dizziness
|
Some examples of safety phrase combinations:
| S3/7/9
| Keep container tightly closed in a cool, well-ventilated place
|
| S3/9/14
| Keep in a cool, well-ventilated place away from... (incompatible materials to be indicated by the manufacturer)
|
| S3/9/49
| Keep only in the original container in a cool, well-ventilated place
|
| S3/14
| Keep in a cool place away from... (incompatible materials to be indicated by the manufacturer)
|
| S7/8
| Keep container tightly closed and dry
|
| S8/10
| Keep container wet, but keep the contents dry
|
| S20/21
| When using do not eat, drink or smoke
|
| S24/25
| Avoid contact with skin and eyes
|
| S27/28
| After contact with skin, take off immediately all contaminated clothing, and wash immediately with plenty of... (to be specified by the manufacturer)
|
| S29/35
| Do not empty into drains; dispose of this material and its container in a safe way
|
| S29/56
| Do not empty into drains, dispose of this material and its container at hazardous or special waste collection point
|
| S36/37/39
| Wear suitable protective clothing, gloves and eye/face protection
|
| S38/2
| In case of insufficient ventilation wear suitable respiratory equipment and stay away from children
|
| S47/49
| Keep only in the original container at temperature not exceeding... °C (to be specified by the manufacturer)
|
| S53
| Avoid exposure - obtain special instructions before use
|
| S56
| Dispose of this material and its container at hazardous or special waste collection point
|
| S57
| Use appropriate containment to avoid environmental contamination
|
6. Find the English equivalents of these words and phrases in the text:
взрывчатый; воздействие; воспламенение; горючий материал; загрязнение окружающей среды; защитная спецодежда; избегать воздействия; информация о мерах предосторожности; канализационные стоки; маркировка; неблагоприятное воздействие; недостаточный; несовместимый; обратиться за медицинской помощью; озоновый слой; описания видов опасного воздействия; паровоздушная смесь; получать; превышающий; предотвращение распространения; производитель; пункт сбора отходов; система органов дыхания; Согласованная на глобальном уровне система классификации опасности и маркировки химической продукции (СГС); соответствовать; средства защиты органов дыхания; указания по рискам и безопасности; утилизировать.
GRAMMAR REVISION.
THE PASSIVE VOICE.
1. Переведите следующие предложения, обращая внимание на:
При следующих глаголах, употребленных в форме страдательного залога, подлежащее английского предложения следует переводить существительным в косвенном падеже (дано только то значение глаголов, которое они имеют в страдательном залоге). Выучите их наизусть.
| ask
| спрашивать
|
| assist
| помогать, содействовать
|
| avoid
| избегать
|
| discuss
| обсуждать
|
| forbid
| запрещать что-либо
|
| give
| давать, приводить к
|
| help
| помогать, содействовать
|
| inform
| сообщать, уведомлять
|
| neglect
| пренебрегать
|
| order
| приказывать, заказывать
|
| precede
| предшествовать
|
| promise
| обещать
|
| refuse
| отказывать
|
| show
| показывать
|
THE INFINITIVE.
Запомните значения следующих союзов, наречий и прилагательных, с которыми соотносится инфинитив в функции обстоятельств 1) цели и 2) следствия:
| 1)
| in order (to)
| для того, чтобы
|
|
| so as
| так чтобы; с тем чтобы
|
| 2)
| so… as (to)
| так (такой, настолько)…, чтобы
|
|
| such …as (to)
| такой … что
|
|
| too
| слишком (перед прилагательным)
|
|
| enough
| достаточно
|
|
| sufficiently
| достаточно
|
|
| sufficient
| достаточный
|
THE PARTICIPLE.
THE GERUND.
Запомните значения глаголов, после которых прямое дополнение может употребляться в форме герундия:
THE MODAL VERBS
may (might), can (could), ought, must, need
СПИСОК ЛИТЕРАТУРЫ
1. Близниченко К.Л., Прусс Н.М. Английский язык. Пособие для вечерних и заочных отделений химико-технологических вузов. – М.: Высш.шк., 1991. – 144 с.
2. Даминова С.О., Леенсон И.А. Англо-русский словарь сокращений в химии. Учебное пособие./ЛИБРОКОМ.- Москва, 2016 – 192с.
3. Даминова С.О., Леенсон И.А. Англо-русский словарь химического лабораторного оборудования./ЛИБРОКОМ.- Москва, 2010 – 208с.
4. Кутепова, М. The World of Chemistry/Английский язык для химиков – КДУ – М,2013
5. Михельсон Т.Н., Успенская Н.В. Практический курс грамматики английского языка. – Санкт-Петербург: Специальная литература, 1995. – 256 с.
6. Онлайн ресурс About Education [Электронный ресурс]. – URL: http://chemistry.about.com (Дата обращения: 12.09.2016).
7. Онлайн ресурс Elements Database [Электронный ресурс]. – URL: http://www.elementsdatabase.com (Дата обращения: 12.09.2016).
8. Онлайн ресурс Encyclopedia Britannica [Электронный ресурс]. – https://global.britannica.com (Дата обращения: 20.08.2016).
9. Онлайн ресурс English for Chemistry & Materials Science [Электронный ресурс]. – URL: https://chemistryenglish.wordpress.com (Дата обращения: 02.08.2016).
10. Онлайн ресурс Life Science [Электронный ресурс]. – URL: http://www.livescience.com (Дата обращения: 18.08.2016).
11. Онлайн ресурс Los Alamos National Laboratory [Электронный ресурс]. – URL: http://www.lanl.gov/ (Дата обращения: 11.09.2016).
12. Онлайн ресурс Science world [Электронный ресурс]. – URL: http://scienceworld.wolfram.com (