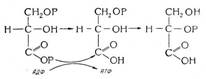
Our modern day periodic table is expanded beyond Mendeleev's initial 63 elements. Most of the current periodic tables include 108 or 109 elements.
The modern periodic table of the elements contains 18 _______, or vertical columns. Elements in a _______ have similar chemical and physical properties because they have the same number of outer electrons. Elements in a _______ are like members of a family - each is different, but all are related by common characteristics.
Each of the table's horizontal rows is called a _______. Along a ___________ a gradual change in chemical properties occurs from one element to another. For example, metallic properties decrease and nonmetallic properties increase as you go from left to right. The periodic table consists of seven _______, which vary in length. The first one is very short and contains only 2 elements, hydrogen and helium, while the sixth has 32 elements. The last ______ is not complete yet because new exotic or manmade elements are still being made in laboratories.
Many _______ are easily recognized by non-chemists. Common examples are copper, lead, silver and gold. In general, _______ have a luster, are quite dense, and are good conductors of heat and electricity. They tend to be soft and malleable (meaning that they are easily shaped and can be drawn into fine wires without breaking)
The ______________ show a closer relationship in their properties than do any other family of elements in the periodic table. They are so chemically reactive that they are never found in the element form in nature. All these metals react spontaneously with gases in the air. They are so soft that they can be cut with an ordinary table knife, revealing a very "buttery", silvery metal surface that immediately turns dull as it reacts with water vapor and oxygen in the air. The chemical reactivity of ________ increases as the atomic number increases.
The ________ also exhibit the typical metal characteristics of high density, metallic luster and electrical and thermal conductivity. Radium, the largest of them, is a radioactive element that occurs naturally only in very small quantities.
The ____________(or heavy) have most of the usual properties of metals. One of their main uses is the formation of alloys to produce tools and construction materials for specific uses. Copper, silver and gold are sometimes known as coinage metals because they can be found naturally in the free state and because they tarnish slowly. Although many _____________ have very high melting and boiling points, mercury has such a low melting point that it is a liquid at room temperature. All _____________are electrical conductors, with copper, silver and gold being among the best
The __________ consist of the lanthanide series and the actinide series. They got their name because they are difficult to find. They often appear to be an add-on to the rest of the periodic table, but actually, they should be shown in the center of the table. They are all unstable. An unstable isotope of an element decays or disintegrates spontaneously, emitting various types of radiation. In some instances, the decay process is slow, with the unstable atom lasting days or months. In others, the process is rapid, lasting tiny fractions of a second. In addition to radiation, the unstable element changes its nucleus to become one or more other lighter elements.
____________ have some of the properties of metals and nonmetals. A few are shiny like metals, but do not really have a metallic luster. Some _______ have very high melting and boiling points; others do not. Others conduct electricity, but their electrons are mobile in only certain directions, so they are called semi-conductors.
The _______________ are in the upper right portion of the periodic table. At room temperature and pressure, many of them exist as gases, but one is a liquid. Others are either the hardest or the softest of solids. The ______ have few chemical properties in common. They range from fluorine, the most active ___________, to the most nonreactive of the elements, the noble gases. Millions of compounds formed from carbon, hydrogen, oxygen, sulfur and nitrogen are known as organic chemicals.
The ____________ are the gases which name indicates that they are salt-formers. This is appropriate since they react easily with other elements, especially the alkali and alkaline earth elements in the left columns of the periodic table. They are all considered highly reactive elements.
The elements are termed _________ because they do not interact with other elements to form compounds. Another way to say this is that they are inert. Their atoms do not even interact with each other, so they exist as mono atomic gases.
10. Comprehension check. Decide if the following statements are true or false. Correct the false ones:
1. Vertical columns in the periodic table are called periods and horizontal rows are called groups.
2. All the elements of the same period demonstrate similar properties.
3. Each period has a different number of elements in it.
4. Alkali metals are among the hardest solids on the earth.
5. Alkali metals can’t be found naturally in the free state, because of their high reactivity.
5. Radium belongs to transition metal group.
6. All metals are solids under normal conditions.
7. Not all the transition metals conduct the electricity well.
8. Rare earth metals were named so, because it is hard to find them on our planet.
9. You can find rare earth metals right in the middle of the periodic table.
10. Rare earth metals are unstable and form heavier elements after they decay.
11. It is difficult to find similar properties for metalloids.
12. All nonmetals can form organic compounds.
13. The name of halogens is connected with their common ability to form particular compounds.
14. Noble gases react only with the elements of their own group.
11. Study Text B and find the English adequate equivalents of the following words and phrases:
1) постепенное изменение; 2) состоит из; 3) встречается в природе; 4) в несвязанном состоянии; 5) при комнатной температуре; 6) излучать радиацию; 7) разложение; 8) длиться доли секунды; 8) взаимодействовать друг с другом.