Kinetic considerations. Changes of phase in the solid state involve a redistribution of the atoms in that solid and the kinetics of the change necessarily depend upon the rate of atomic migration. The transport of atoms through the crystal is more generally termed diffusion. This can occur more easily with the aid of vacancies, since the basic act of diffusion is the movement of an atom to an empty adjacent atomic site.
Let us consider that during a phase change an atom is moved from an α-phase lattice site to a more favourable β-phase lattice site. The energy of the atom should vary with distance as shown in Figure 3.45, where the potential barrier which has to be overcome arises from the interatomic forces between the moving atom and the group of atoms which adjoin it and the new site. Only those atoms (n) with an energy greater than Q are able to make the jump, where 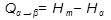 and
and  are the activation enthalpies for heating and cooling, respectively. The probability of an atom having sufficient energy to jump the barrier is given, from the Maxwell–Boltzmann distribution law, as proportional to exp [- Q/ k T] where k is Boltzmann’s constant, T is the temperature and Q is usually expressed as the energy per atom in electron volts.
are the activation enthalpies for heating and cooling, respectively. The probability of an atom having sufficient energy to jump the barrier is given, from the Maxwell–Boltzmann distribution law, as proportional to exp [- Q/ k T] where k is Boltzmann’s constant, T is the temperature and Q is usually expressed as the energy per atom in electron volts.
The rate of reaction is given by Rate = A exp [- Q/ k T] (3.8) where A is a constant involving n and v, the frequency of vibration. To determine Q experimentally, the reaction velocity is measured at different temperatures and, since ln (Rate) = ln A - Q/ k T (3.9) the slope of the In (rate) versus 1/T curve gives Q/ k. In deriving equation (3.8), usually called an Arrhenius equation after the Swedish chemist who first studied reaction kinetics, no account is taken of the entropy of activation, i.e. the change in entropy as a result of the transition. In considering a general reaction the probability expression should be written in terms of the free energy of activation per atom F or G rather than just the internal energy or enthalpy.
The rate equation then becomes Rate = A exp [- F/ k T] = A exp [S/ k ] exp[- E/ k T] (3.10). The slope of the ln (rate) versus 1/T curve then gives the temperature-dependence of the reaction rate, which is governed by the activation energy or enthalpy, and the magnitude of the intercept on the ln (rate) axis depends on the temperature-independent terms and include the frequency factor and the entropy term.
During the transformation it is not necessary for the entire system to go from α to β at one jump and, in fact, if this were necessary, phase changes would practically never occur. Instead, most phase changes occur by a process of nucleation and growth. Chance thermal fluctuations provide a small number of atoms with sufficient activation energy to break away from the matrix (the old structure) and form a small nucleus of the new phase, which then grows at the expense of the matrix until the whole structure is transformed. By this mechanism, the amount of material in the intermediate configuration of higher free energy is kept to a minimum, as it is localized into atomically thin layers at the interface between the phases. Because of this mechanism of transformation, the factors which determine the rate of phase change are: (1) the rate of nucleation, N (i.e. the number of nuclei formed in unit volume in unit time) and (2) the rate of growth, G (i.e. the rate of increase in radius with time). Both processes require activation energies, which in general are not equal, but the values are much smaller than that needed to change the whole structure from α to β in one operation. Even with such an economical process as nucleation and growth transformation, difficulties occur and it is common to find that the transformation temperature, even under the best experimental conditions, is slightly higher on heating than on cooling. This sluggishness of the transformation is known as hysteresis, and is attributed to the difficulties of nucleation, since diffusion, which controls the growth process, is usually high at temperatures near the transformation temperature and is, therefore, not rate-controlling. Perhaps the simplest phase change to indicate this is the solidification of a liquid metal.
5. Fill in the prepositions (of, through, with, from, to, as) where necessary:
1. The transport ___ atoms ___ the crystal is more generally termed diffusion.
2. This can occur more easily ___ the aid ___ vacancies.
3. During a phase change an atom is moved ___ an α-phase lattice site ___ a more favourable β-phase lattice site.
4. Even with such an economical process ___ nucleation and growth transformation, difficulties occur.
5. This sluggishness ___ the transformation is known ___ hysteresis, and is attributed ___ the difficulties of nucleation.
6. Chance thermal fluctuations provide a small number ___ atoms with sufficient activation energy to break away ___ the matrix (the old structure) and form a small nucleus ___ the new phase.
6. Join the halves of the sentences in the columns:
| During the transformation it is not necessary for
| a process of nucleation and growth.
|
| Most phase changes occur by
| which in general are not equal.
|
| Both processes require activation energies,
| is known as hysteresis.
|
| This sluggishness of the transformation
| the entire system to go from α to β at one jump.
|
7. Answer the questions:
What is termed diffusion?



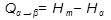 and
and  are the activation enthalpies for heating and cooling, respectively. The probability of an atom having sufficient energy to jump the barrier is given, from the Maxwell–Boltzmann distribution law, as proportional to exp [- Q/ k T] where k is Boltzmann’s constant, T is the temperature and Q is usually expressed as the energy per atom in electron volts.
are the activation enthalpies for heating and cooling, respectively. The probability of an atom having sufficient energy to jump the barrier is given, from the Maxwell–Boltzmann distribution law, as proportional to exp [- Q/ k T] where k is Boltzmann’s constant, T is the temperature and Q is usually expressed as the energy per atom in electron volts.