
Наброски и зарисовки растений, плодов, цветов: Освоить конструктивное построение структуры дерева через зарисовки отдельных деревьев, группы деревьев...

Кормораздатчик мобильный электрифицированный: схема и процесс работы устройства...

Наброски и зарисовки растений, плодов, цветов: Освоить конструктивное построение структуры дерева через зарисовки отдельных деревьев, группы деревьев...

Кормораздатчик мобильный электрифицированный: схема и процесс работы устройства...
Топ:
Методика измерений сопротивления растеканию тока анодного заземления: Анодный заземлитель (анод) – проводник, погруженный в электролитическую среду (грунт, раствор электролита) и подключенный к положительному...
Характеристика АТП и сварочно-жестяницкого участка: Транспорт в настоящее время является одной из важнейших отраслей народного...
Характеристика АТП и сварочно-жестяницкого участка: Транспорт в настоящее время является одной из важнейших отраслей народного хозяйства...
Интересное:
Наиболее распространенные виды рака: Раковая опухоль — это самостоятельное новообразование, которое может возникнуть и от повышенного давления...
Берегоукрепление оползневых склонов: На прибрежных склонах основной причиной развития оползневых процессов является подмыв водами рек естественных склонов...
Отражение на счетах бухгалтерского учета процесса приобретения: Процесс заготовления представляет систему экономических событий, включающих приобретение организацией у поставщиков сырья...
Дисциплины:
|
из
5.00
|
Заказать работу |
Содержание книги
Поиск на нашем сайте
|
|
|
|
1 The term ‘yield point’ is applicable only to low-carbon steels.
2 Ductility denotes the capacity to be drawn from a larger to a smaller diameter of wire.
3 Brittleness means the capacity to be rolled or hammered into thin sheets.
4 Often ductile metals are brittle.
5 Hardness may be defined as resistance to indentation.
6. Answer the questions:
What point of stress-strain curve does yield point refer to?
What grades of steels is yield point applicable to? Why?
What characteristics are used as a measure of the ductility of the metal?
What property of metal is characterized by toughness?
Which test is commonly used to measure toughness?
What properties does a malleable metal possess?
Is there a difference between malleability and ductility?
How does brittleness correlate with toughness and ductility?
9 What does ‘alternation of stress’ mean?
Why is hardness difficult to define?
How is hardness usually characterized and why?
SECTION II
Unit I. PRINCIPLES AND APPLICATIONS OF PHASE DIAGRAMS
Part I
1. Learn the words:
| to refer | относиться |
| separate | отдельный |
| identifiable | опознаваемый |
| substance | вещество |
| to exist | существовать |
| applicable to | применимый к |
| to provide | предоставлять |
| convenient | удобный |
| in addition to | в дополнении к |
| iron | железо |
| refractory oxide silica | огнеупорный оксид кремния |
| tridymite | тридимит |
| cristobalite | кристобалит |
| silica glass | кварцевое стекло |
| molten silica | расплавленный кремнезем |
| glass-reinforced polymer | армированный стекловолокном полимер |
| fibreglass | стекловолокно |
| bounding surface | ограничивающая поверхность |
| contiguous phase | непрерывная фаза |
| structural disturbances and imperfections | структурные нарушения и недостатки |
| an oxide solid solution | твердый раствор оксида |
| by convention | условно |
| voids | пустоты |
| rapid cooling rates | скорость быстрого охлаждения |
| equilibrium | равновесие |
| annealing of metals and alloys | отжиг металлов и сплавов |
| structural heterogeneity | структурная неоднородность |
| high silica content | высокое содержание кремнезема |
| boric oxide | оксид бора |
| a porous siliceous matrix | пористая кремниевая матрица |
| withstand quenching | выдерживать закалку |
2. Guess the meaning of the following words and word combinations:
Microdefects; grain boundaries; metastable condition; ‘triggering’ process; nonequilibrium; scientific difficulty; high temperature applications; to be heat-treated; separation of two phases; electron microscopy; boron-rich phase; skeletal structure; a low linear coefficient; thermal expansion; iced water; maximum service temperature.
3. Identify the part of speech and translate the words:
To separate, separation, separately; to identify, identification, identifier; to exist, existence, existing; to apply, application, applicable; to provide, provider, providing; to differentiate, differentiation, differential; to imply, implied, implicit; to reveal, revealing; to surround, surrounded, surroundings; consolidate, consolidation; to refer, reference; to express, expression, expressed; to add, additional, addition; to comprise, comprising; to disturb, disturbance, disturbed.
4. Match the words with their definitions:
| the concept of a phase | чистый металл |
| crystalline and non-crystalline materials | тепловая активация |
| way of expressing | термостойкий |
| exist as a liquid or vapour | вязкость |
| a pure metal | процесс изготовления |
| thermal activation | существовать в виде жидкости или пара |
| thermal-shock resistant | способ выражения |
| viscosity | кристаллические и некристаллические материалы |
| fabrication process | концепция фазы |
Text A
The concept of a phase
The term ‘phase’ refers to a separate and identifiable state of matter in which a given substance may exist. Being applicable to both crystalline and noncrystalline materials, its use provides a convenient way of expressing a material’s structure. Thus, in addition to the three crystalline forms, the element iron may exist as a liquid or vapour, giving five phases overall. Similarly, the important refractory oxide silica is able to exist as three crystalline phases, quartz, tridymite and cristobalite, as well as a non-crystalline phase, silica glass, and as molten silica. Under certain conditions, it is possible for two or more different phases to co-exist. The glass-reinforced polymer (GRP) known as Fibreglass is an example of a two-phase structure. When referring to a particular phase in the structure of a material, we imply a region comprising a large number of atoms (or ions or molecules) and the existence of a bounding surface which separates it from contiguous phases. Local structural disturbances and imperfections are disregarded. Thus, a pure metal or an oxide solid solution are each described, by convention, as single-phase structures even though they may contain grain boundaries, concentration gradients (coring) and microdefects, such as vacancies, dislocations and voids. Industrial practice understandably favours relatively rapid cooling rates, frequently causing phases to exist in a metastable condition. Some form of ‘triggering’ process, such as thermal activation, is needed before a metastable phase can adopt the stable, or equilibrium, state of lowest energy (e.g. annealing of metals and alloys). These two features, structural heterogeneity on a micro-scale and nonequilibrium, do not give rise to any untoward scientific difficulty.
The production of the thermal-shock resistant known as Vycor provides an interesting example of the potential of phase control. Although glasses of very high silica content are eminently suitable for high temperature applications, their viscosity is very high, making fabrication by conventional methods difficult and costly. This problem was overcome by taking advantage of phase separation in a workable silica containing a high proportion of boric oxide. After shaping, this glass is heat-treated at a temperature of 500–600°C in order to induce separation of two distinct and interpenetrating glassy phases. Electron microscopy reveals a wormlike boron-rich phase surrounded by a porous siliceous matrix. Leaching in hot acid dissolves away the former phase, leaving a porous silica-rich structure. Consolidation heat-treatment at a temperature of 1000°C ‘shrinks’ this skeletal structure by a remarkable 30%. This product has a low linear coefficient of thermal expansion and can withstand quenching into iced water from a temperature of 900°C, its maximum service temperature.
5. Open the brackets and put the predicate in the correct form, translate the sentences:
|
|
|
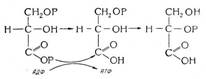
Биохимия спиртового брожения: Основу технологии получения пива составляет спиртовое брожение, - при котором сахар превращается...
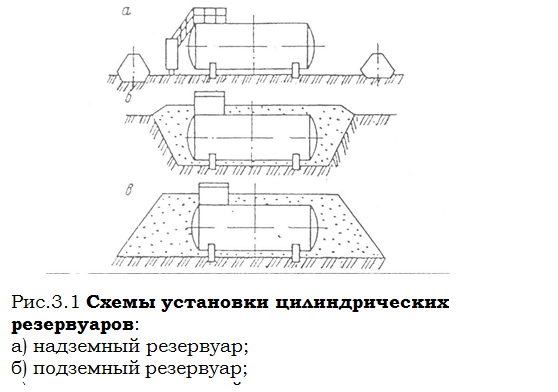
История развития хранилищ для нефти: Первые склады нефти появились в XVII веке. Они представляли собой землянные ямы-амбара глубиной 4…5 м...
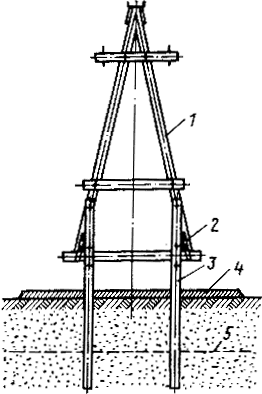
Особенности сооружения опор в сложных условиях: Сооружение ВЛ в районах с суровыми климатическими и тяжелыми геологическими условиями...

Индивидуальные очистные сооружения: К классу индивидуальных очистных сооружений относят сооружения, пропускная способность которых...
© cyberpedia.su 2017-2026 - Не является автором материалов. Исключительное право сохранено за автором текста.
Если вы не хотите, чтобы данный материал был у нас на сайте, перейдите по ссылке: Нарушение авторских прав. Мы поможем в написании вашей работы!