
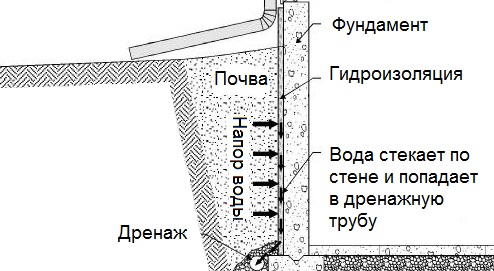
Общие условия выбора системы дренажа: Система дренажа выбирается в зависимости от характера защищаемого...
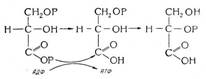
Биохимия спиртового брожения: Основу технологии получения пива составляет спиртовое брожение, - при котором сахар превращается...

Общие условия выбора системы дренажа: Система дренажа выбирается в зависимости от характера защищаемого...

Биохимия спиртового брожения: Основу технологии получения пива составляет спиртовое брожение, - при котором сахар превращается...
Топ:
Оценка эффективности инструментов коммуникационной политики: Внешние коммуникации - обмен информацией между организацией и её внешней средой...
Основы обеспечения единства измерений: Обеспечение единства измерений - деятельность метрологических служб, направленная на достижение...
Организация стока поверхностных вод: Наибольшее количество влаги на земном шаре испаряется с поверхности морей и океанов...
Интересное:
Берегоукрепление оползневых склонов: На прибрежных склонах основной причиной развития оползневых процессов является подмыв водами рек естественных склонов...
Аура как энергетическое поле: многослойную ауру человека можно представить себе подобным...
Распространение рака на другие отдаленные от желудка органы: Характерных симптомов рака желудка не существует. Выраженные симптомы появляются, когда опухоль...
Дисциплины:
|
из
5.00
|
Заказать работу |
Содержание книги
Поиск на нашем сайте
|
|
|
|
Alimkulova E.
Studing-methodical complex
on discipline: chemistry

Astana 2014
| It is considered and is recommended at meeting of the methodical commission of S. Seifullin Kazakh AgroTechnical University "__" _____ 2014, Protocol № __ | First vice rector, Professor Abdyrov A.M. "__" ____ 2014, Protocol № __ |
Author: Candidate of Pedagogika, PhD of chair of physics and chemistry Alimkulova E.J
Reviewer: Makish G.- Candidate of Chemical Sciences
The tutorial comprises theoretical material, description of laboratory works on chemistry. The tutorial is intended for student of the higher thehnical school of face-to-face learning, technical specialities in science and technology. The educational-methocal complex contains: syllabus, a course of lectures, laboratory workshop, set for independent work of students, control-measuring equipment, references and appendix.
Educational and methodical complex it is intended for students of specialty 5B0702400 – «Techical machinery and equipment»
Discussed at the meeting of the chair of chemistry and physics
"__" ___________ 2014, Protocol № __
It is considered and is recommended at meeting of the methodical commission of faculty of Computer systems and Vocational education
"__" ___________ 2014, Protocol № __
S.Seifullin Kazakh Agro Technical University, 2014
TABLE OF CONTENTS
| Page | ||
| Syllabus | 3 | |
| Preface | 11 | |
| Clossary | 15 | |
| Chemistry lab safety | 24 | |
| BASIC CONCEPTS AND LAWS OF CHEMISTRY The structure of an atom. The periodic law of D.I. Mendeleev | 26 | |
| Chemical Bonding | 59 | |
| pH Calculations | 63 | |
| Chemistry of neutralization and hidrolysis | 66 | |
| Chemical Thermodynamics | 68 | |
| Chemical Kinetics | 75 | |
| Coordination complex | ||
| Oxidation-Reduction Reactions | 78 | |
| Test questions | 115 | |
| subject of papers | 144 | |
| Supplement | 146 |
Discipline “ Chemistry ”
1. Teachers’ data: Alimkulova Elmira- Candidate of Pedagogical Sciences, Associate Professor
Scientific interests: Teaching ways of education and upbrining
Telephone: 38-96-46
Classroom: 0402
Time of office hours: 8.15 – 18.00
2. “Chemistry” discipline’s data:
The credits’ quantity - 1
Number of lectures - 15
Number of labs – 15
Number of self-independent work – 30
Class hours’ distribution
Distribution of credit hours
| Weeks | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Total |
| Lecture | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Practical classes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Office hours | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Self-study | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 45 |
| Total | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 90 |
3. Pre-requisites: Chemistry (basics), Mathematics, Physics, and related technical disciplines of the specialty.
4. Pre-requisities of discipline:
- Physics;
- Mathematics;
- Inorganic Chemistry;
5. BRIEF COURSE DESCRIPTION
The purpose of the course:
1) to convince the student of the importance of chemistry in life of modern society;
2) to introduce data on a role of chemical knowledge in an intellectual and informative and practical projection to the student;
3) to teach the student to use and "extract" necessary "chemical" information;
4) to acquaint with the main regularities of course of chemical processes;
5) to train in equipment of performance of chemical experiments, registrations of schemes of chemical processes and necessary calculations;
6) to study the most important questions of professional value, use of chemical processes;
Course objectives are: development of chemistry by formation of knowledge of a substance structure, acquaintance with the main classes of inorganic substances, regularities of chemical reactions, the phenomena in solutions and oxidation-reduction processes.
By the end of the course students must be able:
1. To form general idea on the theoretical questions of chemistry important in practice of biotechnologists.
2. To learn to use an additional material in chemistry (D.I.Mendeleyev's table, the table «Solubility of salts and bases», a number of tension of metals, data on const of a dissotsiation, the table of thermodynamic functions).
3. To develop skills of independent work with educational and special literature;
4. To seize equipment of carrying out simple chemical experiments with further generalization of the received results;
5. To know the ways of the solution of various types of settled tasks, be able to make independently chemical experiment with further generalization of the obtained results.
6. COURSE CONTENT
6.1. List of lectures
| Themes | Hours | Reference | Rate score |
| Theme 1. Bases of the nuclear and molecular doctrine. Main concepts and chemistry laws. Law of equivalents. | 1 | 1-3 | 0.3 |
| Theme 2. Structure of atom and periodic law. | 1 | 1-3 | 0.3 |
| Theme 3. Chemical bond and structure of molecules. | 1 | 1-3 | 0.3 |
| Theme 4. Main regularities of course of chemical reactions. Bases of chemical thermodynamics. Thermochemistry. | 1 | 1-3 | 0.3 |
| Theme 5. Kinetics of chemical reactions. Chemical balance. | 1 | 1-3 | 0.3 |
| Theme 6. Disperse systems. True solutions. Solutions of nonelectrolits. | 1 | 1-3 | 0.3 |
| Theme 7. Water solutions of electrolits. Theory of an electrolytic dissociation. Strong and weak electrolits. | 1 | 1-3 | 0.3 |
| Theme 8. Dissociation of water. Hydrogen indicator. Buffer solutions. | 1 | 1-3 | 0.3 |
| Theme 9. Hydrolysis of salts. | 1 | 1-3 | 0.3 |
| Theme 10. Complex connections. | 1 | 1-3 | 0.3 |
| Theme 11. Oxidation-reduction reactions. | 1 | 1-3 | 0.3 |
| Theme 12. Colloidal solutions. | 1 | 1-3 | 0.3 |
| Theme 13-15. Electrochemical Cells. Galvanic and Electrolytic Cells | 3 | 3-5 | 0.9 |
6.2 List of laboratory and practical classes
| №п/п | Themes | Hours | Reference | Control’s rate score |
| 1 | Bases of the nuclear and molecular doctrine. Main concepts and chemistry laws. Law of equivalents. | 1 | 4 | 0,33 |
| 2 | Structure of atom and periodic law. | 1 | 4 | 0,33 |
| 3 | Chemical bond and structure of molecules. | 1 | 4 | 0,33 |
| 4 | Main regularities of course of chemical reactions. Bases of chemical thermodynamics. Thermochemistry. | 1 | 4 | 0,33 |
| 5 | Kinetics of chemical reactions. Chemical balance. | 1 | 4 | 0,33 |
| 6 | Disperse systems. True solutions. Solutions of non electrolits. | 1 | 4 | 0,33 |
| 7 | Water solutions of electrolits. Theory of an electrolytic dissociation. Strong and weak electrolits. | 1 | 4 | 0,33 |
| 8 | Dissociation of water. Hydrogen indicator. Buffer solutions. | 1 | 4 | 0,33 |
| 9 | Hydrolysis of salts. | 1 | 4 | 0,33 |
| 10 | Complex connections. | 1 | 4 | 0,33 |
| 11 | Oxidation-reduction reactions. | 1 | 4 | 0,33 |
| 12-15 | Electrochemical Cells. Galvanic and Electrolytic Cells | 4 | 4 | 0,33*4 |
7. SCHEDULE OF STUDENT’S OUTPUT
| № | Theme | Content (tasks) of self-study | Expected results | Reference | Assessment | Due to… | Score |
| 1 | Fundamental laws of chemistry. | Law of equivalents, gas laws, Avogadro's law. Solution of tasks. | - | 1-3 | Task checking | 1 week | 1 |
| 2 | Mendeleyev's periodic system | To know the periodic law, a system structure. To write electronic and graphic formulae of 4 elements, to characterize their properties. | - | 1-3 | Task checking | 2 week | 1 |
| 3 | Chemical bond | Types of a chemical bond. Structure of molecules. Concept about hybridization. Mechanism of formation of a chemical bond. Hydrogen bond. To make notes. | - | 1-3 | Recitation according to the abstract | 3 week | 1 |
| 4 | Power of chemical processes. | Concepts enthaly, entropy, Gibbs's energy. To be able to define the reaction direction. Solution of problems. | - | 1-3 | Task checking | 4 week | 1 |
| 5 | The kinetics of chemical processes | To Distinguish homogeneous and heterogeneous reactions, to know the factors influencing speed of reactions. Solution of problems. | - | 1-3 | Task checking | 5 week | 1 |
| 6 | Ways of expression of concentration of solutions. | To be able to express concentration of solutions, to pass from one concentration to another. Solution of problems. | - | 1-3 | Task checking | 6 week | 1 |
| 7 | Properties of solutions of non electrolits. | Osmosis, osmotic pressure to be able to define temperature of boiling and freezing of solutions. Raul, Vant-Goff's laws. Solution of problems. | - | 1-3 | Task checking | 7 week | 1 |
| 8 | Solutions of electrolits. | To distinguish strong and weak electrolits. To be able to write down the ionic equations of reactions. To do exercises. | - | 1-3 | Task checking | 8 week | 1 |
| 9 | Hydrogen indicator. | To be able to define рН strong and weak acids and the bases. Solution of problems. | - | 1-3 | Task checking | 9 week | 1 |
| 10 | Hydrolysis of salts. | To be able to define nature of hydrolysis of salt, to write down the reaction equation in an ionic and molecular look. To do exercises. | - | 1-3 | Task checking | 10 week | 1 |
| 10 | Oxidation-reduction reactions. | Concepts: oxidizer reducer, oxidation, restoration. To be able to define painting masses of equivalents of -oxidizers and reducers. Solution of problems. | - | 1-3 | Task checking | 11 week | 1 |
| 11 | Colloidal solutions. | To know a structure and properties of a colloidal particle. To make notes. | - | 1-3 | Verification of abstracts and recitation | 12 week | 1 |
| 12 | Electrochemical Cells. Galvanic and Electrolytic Cells | - | 3-5 | Task checking | 13 week | 1 | |
| 13-15 | Galvanic and Electrolytic Cells | - | 3-5 | Task checking | 14 week | 3 |
8. REFERENCE
1. Glinka N.L.General chemistry.
2. Chemistry. Allan Blackman, Steve Bottle, Siebert Schmid, Mauro Mocerino, Uta Wille.
3. Chemistry. 4th edition. Rob Lewis, Wynne Evans.
4. Laboratory Manual for Principles of General Chemistry. Jo Allan Beran.
5. Analytical chemistry. Gary D. Christian.
6. Periodic Table of the Elements. Ekkehard Flug, Klaus G. Heumann.
9. COURSE POLICY
Students are not allowed to
1. be late for classes
2. miss classes without any reason
3. talk during class by cell phone, chew gum
5. avoid wearing business dress code
6. be impolite with fellow students and teachers
10. KNOWLEDGE ASSESSMENT
Assessment criteria on students' knowledge:
- Knowledge of the basic categories of inorganic chemistry
- Fluency in theoretical materials
- Understanding of the laws of inorganic chemistry
- The ability to solve problems by applying acquired knowledge
11. GRADING SYSTEM
The policy of assessment is based on a 100-point system and provides the following distribution of points: the current and intermediate control is assigned a total of 60 points, the final control - 40 points.
11.1 Approximate scheme of knowledge assessment during the course
| № | The student’s activity on: | Number of points min / max |
| I | CURRENT CONTROL: - lecture - workshops - office hours - self-study | 2,5/5 (0,3*15 week =5) 7,5/15 (1*15 week =15) 5/10 (0,7*15 week =10) 5/10 (0,7*15 week =10) |
| II | INTERMEDIATE CONTROL: two of 10 control points for each coursework in the semester | 10/20 (10*2=20) 5 /10 |
| TOTAL: | 30/60 points | |
| III | FINAL EXAM | 20/40 |
| Total | 50/100 points |
11.2 Approximate scheme of the student’s grading at the exam
| № | Examination results | Evaluation in points ( в %) |
| 1. | Current control | 40 |
| 2. | Intermediate control | 20 |
| 3. | Final control | 60 |
11.3 The student’s knowledge assessment scale
| Scores in alphabetic system | The digital equivalent of the point | The percentage of points |
Point on conventional system | |
| А | 4,0 | 95-100 | excellent | |
| А- | 3,67 | 90-94 | ||
| В+ | 3,33 | 85-89 | good | |
| В | 3,0 | 80-84 | ||
| В- | 2,67 | 75-79 | ||
| С+ | 2,33 | 70-74 | satisfactory | |
| С | 2,0 | 65-69 | ||
| С- | 1,67 | 60-64 | ||
| Д+ | 1,33 | 55-59 | ||
| Д | 1,0 | 50-54 | ||
| F | 0 | 0-49 | unsatisfactory | |
Preface
Chemistry is the study of matter and energy and the interactions between them. Chemistry tends to focus on the properties of substances and the interactions between different types of matter, particularly reactions that involve electrons.
Because understanding chemistry helps you to understand the world around you. Cooking is chemistry. Everything you can touch or taste or smell is a chemical. When you study chemistry, you come to understand a bit about how things work. Chemistry isn't secret knowledge, useless to anyone but a scientist. It's the explanation for everyday things, like why laundry detergent works better in hot water or how baking soda works or why not all pain relievers work equally well on a headache. If you know some chemistry, you can make educated choices about everyday products that you use.
Chemistry affects all aspects of life and chemists are therefore involved in tackling major problems of scientific and social concern. It plays an important part in many fields of technology, so that graduate chemists must be familiar with a variety of facts, figures, theories, experimental and instrumental techniques. Its concepts and problem solving opportunities, and its laboratory activates train hand and mind. These and its relation to other sciences from physics to medicine, and its role throughout technology means that graduate chemists have an especially wide choice of career.
You could use chemistry in most fields, but it's commonly seen in the sciences and in medicine. Chemists, physicists, biologists, and engineers study chemistry. Doctors, nurses, pharmacists and veterinarians all take chemistry courses
Chemistry
• The branch of science that deals with the properties of substances and the reactions that transform them into other substances.
• Chemistry is a central science.
• Chemistry uses the scientific method to advance its knowledge.
|
|
|

История создания датчика движения: Первый прибор для обнаружения движения был изобретен немецким физиком Генрихом Герцем...

Своеобразие русской архитектуры: Основной материал – дерево – быстрота постройки, но недолговечность и необходимость деления...
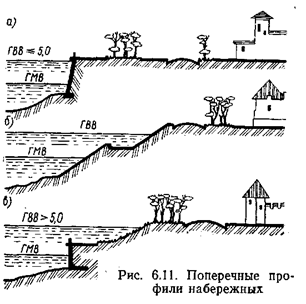
Поперечные профили набережных и береговой полосы: На городских территориях берегоукрепление проектируют с учетом технических и экономических требований, но особое значение придают эстетическим...

Таксономические единицы (категории) растений: Каждая система классификации состоит из определённых соподчиненных друг другу...
© cyberpedia.su 2017-2025 - Не является автором материалов. Исключительное право сохранено за автором текста.
Если вы не хотите, чтобы данный материал был у нас на сайте, перейдите по ссылке: Нарушение авторских прав. Мы поможем в написании вашей работы!