

Таксономические единицы (категории) растений: Каждая система классификации состоит из определённых соподчиненных друг другу...

Кормораздатчик мобильный электрифицированный: схема и процесс работы устройства...

Таксономические единицы (категории) растений: Каждая система классификации состоит из определённых соподчиненных друг другу...

Кормораздатчик мобильный электрифицированный: схема и процесс работы устройства...
Топ:
Проблема типологии научных революций: Глобальные научные революции и типы научной рациональности...
Определение места расположения распределительного центра: Фирма реализует продукцию на рынках сбыта и имеет постоянных поставщиков в разных регионах. Увеличение объема продаж...
Марксистская теория происхождения государства: По мнению Маркса и Энгельса, в основе развития общества, происходящих в нем изменений лежит...
Интересное:
Как мы говорим и как мы слушаем: общение можно сравнить с огромным зонтиком, под которым скрыто все...
Берегоукрепление оползневых склонов: На прибрежных склонах основной причиной развития оползневых процессов является подмыв водами рек естественных склонов...
Инженерная защита территорий, зданий и сооружений от опасных геологических процессов: Изучение оползневых явлений, оценка устойчивости склонов и проектирование противооползневых сооружений — актуальнейшие задачи, стоящие перед отечественными...
Дисциплины:
|
из
5.00
|
Заказать работу |
|
|
|
|
In presentations of the periodic table, the lanthanides and the actinides are customarily shown as two additional rows below the main body of the table,[21]with placeholders or else a selected single element of each series (either lanthanum or lutetium, and either actinium or lawrencium, respectively) shown in a single cell of the main table, between barium and hafnium, and radium and rutherfordium, respectively. This convention is entirely a matter of aesthetics and formatting practicality; a rarely used wide-formatted periodic table inserts the lanthanide and actinide series in their proper places, as parts of the table's sixth and seventh rows (periods).
Some periodic tables include a dividing line, or equivalent, between metals and nonmetals.[22] Various other categories of elementsmay also be highlighted on a periodic table including, for example, transition metals, post-transition metals, or metalloids.[23]Specialized groupings such as the refractory metals and the noble metals, which are subsets (in this example) of the transition metals, are also known and occasionally denoted.
Electron configuration

Approximate order in which shells and subshells are arranged by increasing energy according to theMadelung rule.
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered shell 1, shell 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram to the right. The electron configuration for neon, for example, is 1s2 2s2 2p6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell—two in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.
Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group. The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period. These trends of the atomic radii (and of various other chemical and physical properties of the elements) can be explained by the electron shell theory of the atom; they provided important evidence for the development and confirmation of quantum theory.
The electrons in the 4f-subshell, which is progressively filled from cerium (Z = 58) to lutetium (Z = 71), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out. The elements immediately following the lanthanides have atomic radii which are smaller than would be expected and which are almost identical to the atomic radii of the elements immediately above them.[29] Hence hafnium has virtually the same atomic radius (and chemistry) as zirconium, and tantalum has an atomic radius similar to niobium, and so forth. This is known as the lanthanide contraction. The effect of the lanthanide contraction is noticeable up toplatinum (Z = 78), after which it is masked by a relativistic effect known as the inert pair effect. The d-block contraction, which is a similar effect between the d-block and p-block, is less pronounced than the lanthanide contraction but arises from a similar cause.
|
|
Ionization energy
The first ionization energy is the energy it takes to remove one electron from an atom, the second ionization energy is the energy it takes to remove a second electron from the atom, and so on. For a given atom, successive ionization energies increase with the degree of ionization. For magnesium as an example, the first ionization energy is 738 kJ/mol and the second is 1450 kJ/mol. Electrons in the closer orbitals experience greater forces of electrostatic attraction; thus, their removal requires increasingly more energy. Ionization energy becomes greater up and to the right of the periodic table.
Dependence of electron affinity on atomic number. Values generally increase across each period, culminating with the halogens before decreasing precipitously with the noble gases. Examples of localized peaks seen in hydrogen, the alkali metals and the group 11 elements are caused by a tendency to complete the s-shell (with the 6s shell of gold being further stabilized by relativistic effects and the presence of a filled 4f sub shell). Examples of localized troughs seen in the alkaline earth metals, and nitrogen, phosphorus, manganese and rhenium are caused by filled s-shells, or half-filled p- or d-shells.
The electron affinity of an atom is the amount of energy released when an electron is added to a neutral atom to form a negative ion. Although electron affinity varies greatly, some patterns emerge. Generally, nonmetals have more positive electron affinity values than metals. Chlorine most strongly attracts an extra electron. The electron affinities of the noble gases have not been measured conclusively, so they may or may not have slightly negative values.
Electron affinity generally increases across a period. This is caused by the filling of the valence shell of the atom; a group 17 atom releases more energy than a group 1 atom on gaining an electron because it obtains a filled valence shell and is therefore more stable.
A trend of decreasing electron affinity going down groups would be expected. The additional electron will be entering an orbital farther away from the nucleus. As such this electron would be less attracted to the nucleus and would release less energy when added. However, in going down a group, around one-third of elements are anomalous, with heavier elements having higher electron affinities than their next lighter congenors. Largely, this is due to the poor shielding by d and f electrons. A uniform decrease in electron affinity only applies to group 1 atoms.
Location on the Periodic Table
Metals are located on the left side and the middle of the periodic table. Group IA and Group IIA (the alkali metals) are the most active metals. The transition elements, groups IB to VIIIB, are also considered metals. The basic metals are the element to the right of the transition metals. The bottom two rows of elements beneath the body of the periodic table are the lanthanides and actinides, which are also metals.
|
|
Properties
Metals are shiny solids are room temperature (except mercury, which is a shiny liquid element), with characteristic high melting points and densities. Many of the properties of metals, including large atomic radius, low ionization energy, and low electronegativity, are due to the fact that the electrons in the valence shell of a metal atoms can be removed easily. One characteristic of metals is their ability to be deformed without breaking. Malleability is the ability of a metal to be hammered into shapes. Ductility is the ability of a metal to be drawn into wire. Because the valence electrons can move freely, metals are good heat conductors and electrical conductors.
|
|
|

Папиллярные узоры пальцев рук - маркер спортивных способностей: дерматоглифические признаки формируются на 3-5 месяце беременности, не изменяются в течение жизни...
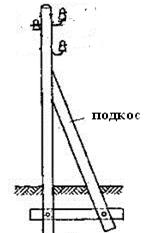
Опора деревянной одностоечной и способы укрепление угловых опор: Опоры ВЛ - конструкции, предназначенные для поддерживания проводов на необходимой высоте над землей, водой...
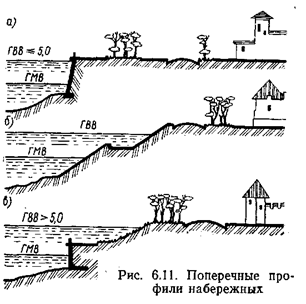
Поперечные профили набережных и береговой полосы: На городских территориях берегоукрепление проектируют с учетом технических и экономических требований, но особое значение придают эстетическим...
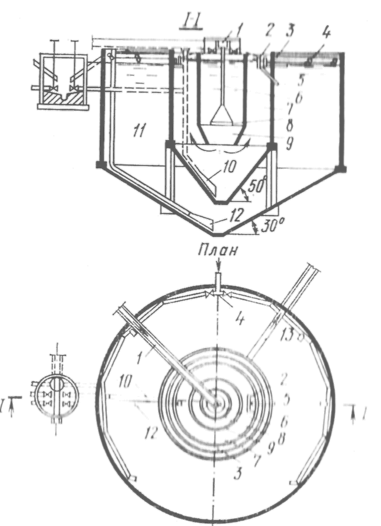
Типы сооружений для обработки осадков: Септиками называются сооружения, в которых одновременно происходят осветление сточной жидкости...
© cyberpedia.su 2017-2024 - Не является автором материалов. Исключительное право сохранено за автором текста.
Если вы не хотите, чтобы данный материал был у нас на сайте, перейдите по ссылке: Нарушение авторских прав. Мы поможем в написании вашей работы!