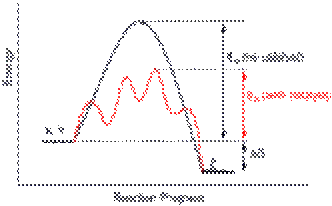
A catalyst permits a different energy pathway for a chemical reaction which has a lower activation energy. The catalyst is not consumed in the chemical reaction.
It's useful to be able to predict whether an action will affect the rate at which a chemical reaction proceeds. There are several factors that can influence the rate of a chemical reaction. In general, a factor that increases the number of collisions between particles will increase the reaction rate and a factor that decreases the number of collisions between particles will decrease the chemical reaction rate.
Concentration of Reactants
A higher concentration of reactants leads to more effective collisions per unit time, which leads to an increasing reaction rate (except for zero order reactions). Similarly, a higher concentation of products tends to be associated with a lower reaction rate. Use the partial pressure of reactants in a gaseous state as a measure of their concentration.
Temperature
Usually, an increase in temperature is accompanied by an increase in the reaction rate. Temperature is a measure of the kinetic energy of a system, so higher temperature implies higher average kinetic energy of molecules and more collisions per unit time. A general rule of thumb for most (not all) chemical reactions is that the rate at which the reaction proceeds will approximately double for each 10°C increase in temperature. Once the temperature reaches a certain point, some of the chemical species may be altered (e.g., denaturing of proteins) and the chemical reaction will slow or stop.
Medium
The rate of a chemical reaction depends on the medium in which the reaction occurs. It may make a difference whether a medium is aqueous or organic; polar or nonpolar; or liquid, solid, or gaseous.
Presence of Catalysts and Competitors
Catalysts (e.g., enzymes) lower the activation energy of a chemical reaction and increase the rate of a chemical reaction without being consumed in the process. Catalysts work by increasing the frequency of collisions between reactants, altering the orientation of reactants so that more collisions are effective, reducing intramolecular bonding within reactant molecules, or donating electron density to the reactants. The presence of a catalyst helps a reaction to proceed more quickly to equilibrium. Aside from catalysts, other chemical species can affect a reaction. The quantity of hydrogen ions (the pH of aqueous solutions) can alter a reaction rate. Other chemical species may compete for a reactant or alter orientation, bonding, electron density, etc., thereby decreasing the rate of a reaction.
Catalysts and Catalysis
A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. The only difference between a catalyzed reaction and an uncatalyzed reaction is that the activation energy is different. There is no effect on the energy of the reactants or the products. The ΔH for the reactions is the same.
Positive and Negative Catalysts
Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst which speeds up the rate of a chemical reaction by lowering its activation energy. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur.
Promoters and Catalytic Poisons
A promoter is a substance that increases the activity of catalyst. A catalytic poison is a substance that inactivates a catalyst.
How Catalysts Work
Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. Note that the catalyst may be consumed during one of the intermediate steps, but it will be created again before the reaction is completed.
Types of Thermodynamic Systems. Internal Energy. The first law of thermodynamics. Enthalpy. Hess’s law. The Second and Third Laws of Thermodynamics. Gibbs Free Energy
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics.
The state functions in chemical thermodynamics:
• Internal energy (DU)
• Enthalpy (D H).
• Entropy (D S)
• Gibbs free energy (D G)
Heat and work are both forms of energy. They are also related forms, in that one can be transformed into the other. Heat energy (such as steam engines) can be used to do work (such as pushing a train down the track). Work can be transformed into heat, such as might be experienced by rubbing your hands together to warm them up.
Thermodynamic systems
Energy transfer is studied in three types of systems:
Open systems can exchange both matter and energy with an outside system. They are portions of larger systems and in intimate contact with the larger system. Your body is an open system.
Closed systems exchange energy but not matter with an outside system. Though they are typically portions of larger systems, they are not in complete contact. The Earth is essentially a closed system; it obtains lots of energy from the Sun but the exchange of matter with the outside is almost zero.
Isolated systems can exchange neither energy nor matter with an outside system. While they may be portions of larger systems, they do not communicate with the outside in any way. The physical universe is an isolated system; a closed thermos bottle is essentially an isolated system (though its insulation is not perfect).
Heat can be transferred between open systems and between closed systems, but not between isolated systems.
Thermodynamic State Properties:
• Extensive: These variables or properties depend on the amount of material present (e.g. mass or volume).
• Intensive: These variables or properties DO NOT depend on the amount of material (e.g. density, pressure, and temperature).
Internal energy (also called thermal energy) is the energy an object or substance is due to the kinetic and potential energies associated with the random motions of all the particles that make it up.
The hotter something is, the faster its molecules are moving or vibrating, and the higher its temperature. Temperature is proportional to the average kinetic energy of the atoms or molecules that make up a substance.
The first law of thermodynamics. Energy and matter can be neither created nor destroyed; only transformed from one form to another. The energy and matter of the universe is constant.
1. When a system changes from one state to another, its internal energy changes.
D U=Ufinal – Uinitial
2. The change in internal energy, DU, equals heat minus work.
D U = q – w
Changes in Enthalpy. Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure. Consider the following expression for a chemical process:
D H = S Hproducts - S Hreactants
If DH >0, then Q <0. The reaction is endothermic
If DH <0, then Q >0. The reaction is exothermic
Hess’s Law states that the heat of a whole reaction is equivalent to the sum of it’s steps.
For example: C + O2 ® CO2
this reaction can occur as 2 steps
1. C + ½O2 ® CO DH° = – 110.5 kJ
CO + ½O2 ® CO2 DH° = – 283.0 kJ
2. C + CO + O2 ® CO + CO2 DH° = – 393.5 kJ
The Second Law of Thermodynamics. In any spontaneous process, there is always an increase in entropy of the universe
D Suniverse = D Ssystem + D Ssurroundings
D Suniverse > 0 for spontaneous rxn
The Third Law of Thermodynamics. The entropy of a perfect crystal at 0 kelvins is zero
D Suniverse = 0 at equilibrium.
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "usefulness" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure (isothermal, isobaric). Just as in mechanics, where potential energy is defined as capacity to do work, similarly different potentials have different meanings. The Gibbs free energy is the maximum amount of non-expansion work that can be extracted from a closed system; this maximum can be attained only in a completely reversible process. When a system changes from a well-defined initial state to a well-defined final state, the Gibbs free energy ΔG equals the work exchanged by the system with its surroundings, minus the work of the pressure forces, during a reversible transformation of the system from the same initial state to the same final state.
D G = D H - T D S
Compares entropy, enthalpy and temperature
• If DG<0 (is negative) the reaction is spontaneous
• If DG> 0 (is positive) the reaction is nonspontaneous
If DG =0 the reaction is at equilibrium
There are four possibilities for the influence that temperature can have on the spontaniety of a process:
1. Under these conditions, both the ΔH and TΔS terms will be negative, so ΔG will be negative regardless of the temperature. An exothermic reaction whose entropy increases will be spontaneous at all temperatures.
2. If the reaction is sufficiently exothermic it can force ΔG negative only at temperatures below which |TΔS| < |ΔH|. The freezing of a liquid or the condensation of a gas are the most common examples of this condition
3. The entropy increase must overcome the handicap of an endothermic process so that TΔS > ΔH. the process will be spontaneous at temperatures above T = ΔH / ΔS. (Think of melting and boiling.)
4. With both ΔH and ΔS working against it, this kind of process will not proceed spontaneously at any temperature. Substance A always has a greater number of accessible energy states, and is therefore always the preferred form.
Hydrogen peroxide decomposes according to the following thermochemical reaction:
H2O2(l) → H2O(l) + 1/2 O2(g); ΔH = -98.2 kJ
Calculate the change in enthalpy, ΔH, when 1.00 g of hydrogen peroxide decomposes.
The thermochemical equation tells us that ΔH for the decomposition of 1 mole of H2O2 is -98.2 kJ, so this relationship can be used as a conversion factor. Using the Periodic Table, the molecular mass of H2O2 is 34.0, which means that 1 mol H2O2 = 34.0 g H2O2.
Using these values:
ΔH = 1.00 g H2O2 x 1 mol H2O2 / 34.0 g H2O2 x -98.2 kJ / 1 mol H2O2
ΔH = -2.89 kJ
Tasks
1. To determine thermal effect of chemical reaction and its size to define process power (endothermic or exothermic).
2. To define entropy change of chemical reaction and draw a conclusion about volume changes during reaction (increases or decreases).
3. To define change of energy of Gibbs at a standard temperature and to draw a conclusion on the direction of spontaneous reaction at a standard temperature (direct or return reaction).
4. To define the reaction direction at the temperature 300 K (direct or return reaction).
5. To determine temperature at which in system there comes an equilibrium state.
| №
| Reaction
| Т,К
|
| 1
| GeCl4(г)+2H2(г)=Ge(к)+4HCl(г)
| 1081
|
| 2
| 2PCl3(г)+3H2(г)=2P(к)+6HCl(г)
| 900
|
| 3
| 2BBr3(г)+3H2(г)=2B(к)+6HBr(г)
| 800
|
| 4
| SiHCl3(г)+H2(г)=Si(к)+3HCl(г)
| 1000
|
| 5
| SiBr4(г)+2H2(г)=Si(к)+4HBr(г)
| 750
|
| 6
| SiCl4(г)+2H2(г)=Si(к)+4HCl(г)
| 1100
|
| 7
| B2H6(г)+3O2(г)=B2O3(к)+3H2O(г)
| 500
|
| 8
| 2P2O5(к)+5Si(к)=5SiO2(к)+4P(к)
| 700
|
| 9
| 4BN(к)+3O2(г)=2B2O3(к)+2N2(г)
| 1200
|
| 10
| 2B2O3(к)+3Si(к)=3SiO2(к)+4B(к)
| 1000
|
| 11
| 3SiBr4(г)+2N2(г)=Si3N4(к)+6Br2(г)
| 850
|
| 12
| SiH4(г)+2O2(г)=SiO2(к)+2H2O(г)
| 300
|
| 13
| SiH4(г)+CO2(г)=SiO2(к)+CH4(г)
| 873
|
| 14
| 3SiH4(г)+4NH3(г)=Si3N4(к)+12H2(г)
| 400
|
| 15
| 3SiH4(г)+6N2H4(г)=Si3N4(к)+8NH3(г)+6H2
| 300
|
| 16
| 3SiCl4(г)+4NH3(г)=Si3N4(к)+12HCl(г)
| 950
|
| 17
| SiH4(г)+2O2(г)=SiO2(к)+2H2O(г)
| 350
|
| 18
| SiCl4(г)+Si(к)=2SiCl2(г)
| 1000
|
| 19
| AsH3(г)+3/2Cl2(г)=As(к)+3HCl(г)
| 450
|
| 20
| SbCl5(к)+5/2H2(г)=Sb(к)+5HCl(г)
| 300
|
| 21
| 2BBr3(г)+3/2O2(г)=B2O5(к)+3Br2(г)
| 500
|
| 22
| 2PCl3(г)+5/2O2(г)=P2O5(к)+3Cl2(г)
| 600
|
| 23
| GeCl4(г)+Ge(к)=2GeCl2(г)
| 973
|
| 24
| Si(к)+2H2O(г)=SiO2(к)+H2(г)
| 1150
|
| 25
| AIF3(к)+3/2H2(г)=Al(к)+3HF(г)
| 783
|
Chemical Kinetics
Explain the term chemical kinetics, and describe factors that influence rate of chemical reactions. Define and use proper units for chemical reaction rates.
Chemical kinetics is the study and discussion of chemical reactions with espect to reaction rates, effect of various variables, re-arrangement of atoms, formation of intermediates etc. There are many topics to be discussed, and each of these topics is a tool for the study of chemical reactions. By the way, the study of motion is called kinetics, from Greek kinesis, meaning movement.
At the macroscopic level, we are interested in amounts reacted, formed, and the rates of their formation. At the molecular or microscopic level, the following considerations must also be made in the discusion of chemical reaction mechanism.
· Molecules or atoms of reactants must collide with each other in chemical reactions.
· The molecules must have sufficient energy (discussed in terms of activation energy) to initiate the reaction.
· In some cases, the orientation of the molecules during the collision must also be considered.
Chemical reaction rates are the rates of change in concentrations or amounts of either reactants or products. For changes in amounts, the units can be one of mol/s, g/s, lb/s, kg/day etc. For changes in concentrations, the units can be one of mol/(L s), g/(L s), %/s etc.
With respect to reaction rates, we may deal with average rates, instantaneous rates, or initial rates depending on the experimental conditions.