
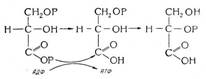
Биохимия спиртового брожения: Основу технологии получения пива составляет спиртовое брожение, - при котором сахар превращается...
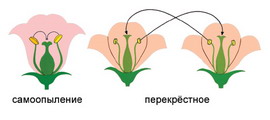
Семя – орган полового размножения и расселения растений: наружи у семян имеется плотный покров – кожура...

Биохимия спиртового брожения: Основу технологии получения пива составляет спиртовое брожение, - при котором сахар превращается...

Семя – орган полового размножения и расселения растений: наружи у семян имеется плотный покров – кожура...
Топ:
Процедура выполнения команд. Рабочий цикл процессора: Функционирование процессора в основном состоит из повторяющихся рабочих циклов, каждый из которых соответствует...
Когда производится ограждение поезда, остановившегося на перегоне: Во всех случаях немедленно должно быть ограждено место препятствия для движения поездов на смежном пути двухпутного...
Эволюция кровеносной системы позвоночных животных: Биологическая эволюция – необратимый процесс исторического развития живой природы...
Интересное:
Наиболее распространенные виды рака: Раковая опухоль — это самостоятельное новообразование, которое может возникнуть и от повышенного давления...
Инженерная защита территорий, зданий и сооружений от опасных геологических процессов: Изучение оползневых явлений, оценка устойчивости склонов и проектирование противооползневых сооружений — актуальнейшие задачи, стоящие перед отечественными...
Берегоукрепление оползневых склонов: На прибрежных склонах основной причиной развития оползневых процессов является подмыв водами рек естественных склонов...
Дисциплины:
|
из
5.00
|
Заказать работу |
|
|
|
|
1. Animals and plants require oxygen for respiration.
2. Oxygen gas is colorless, odorless, and tasteless.
3. Liquid and solid oxygen are pale blue.
4. Oxygen is a non-metal.
5. Oxygen gas normally is the divalent molecule O2. Ozone, O3, is another form of pure oxygen.
6. Oxygen supports combustion.
7. Oxygen is paramagnetic.
8. Approximately 2/3 of the mass of the human body is oxygen.
9. Excited oxygen is responsible for the bright red and yellow-green colors of the aurora.
10.Oxygen was the atomic weight standard for the other elements until 1961 when it was replaced by carbon 12.
You breathe oxygen, yet air is mostly nitrogen. You need nitrogen to live and encounter it in the foods you eat and in many common chemicals. Here are some quick facts about this element. You can find detailed information about nitrogen on the nitrogen facts page.
1. Nitrogen is odorless, tasteless, and colorless.
2. Nitrogen gas (N2) makes up 78.1% of the volume of the Earth's air.
3. Nitrogen is a nonmetal.
4. Nitrogen gas is relatively inert, but soil bacteria can 'fix' nitrogen into a form that plants and animals can use to make amino acids and proteins.
5. The French chemist Antoine Laurent Lavoisier named nitrogen azote, meaning without life.
6. Nitrogen was sometimes referred to as 'burnt' or 'dephlogisticated' air, since air that no longer contains oxygen is almost all nitrogen. The other gases in air are present in much lower concentrations.
7. Nitrogen compounds are found in foods, fertilizers, poisons, and explosives. Your body is 3% nitrogen by weight.
8. Nitrogen is responsible for the orange-red, blue-green, blue-violet, and deep violet colors of the aurora.
9. One way to prepare nitrogen gas is by liquefaction and fractional distillation from the atmosphere.
10.Nitrogen has a valence of 3 or 5.
 Here are some facts about lithium. You can get more detailed information from the Periodic Table entry for lithium.
Here are some facts about lithium. You can get more detailed information from the Periodic Table entry for lithium.
1. Lithium is the third element in the periodic table, with protons and the element symbol Li.
2. Lithium is an alkali metal.
3. Lithium metal burns white, though it imparts a crimson color to a flame.
4. Lithium does not occur free in nature, though it is found in nearly all igneous rocks and in mineral springs.
5. Pure lithium metal is extremely corrosive and requires special handling.
6. Lithium is the lightest metal, with a density about half that of water. In other words, if lithium did not react with water (which it does, somewhat vigorously), it would float.
7. Among other uses, lithium is used in medicine, as a heat transfer agent, for making alloys, and for batteries.
8. The transmutation of lithium to tritium was the first man-made nuclear fusion reaction.
9. The name for lithium comes from Greek lithos which means stone. Lithium occurs in most igneous rocks, although it does not occur free in nature.
10. Lithium metal is made by electrolysis of fused lithium chloride.
|
|
Sodium displays its brilliant yellow emission in this flame test of sodium carbonate.
 Sodium is a silvery-white metal belonging to Group 1 of the Periodic Table, which is the alkali metals group.
Sodium is a silvery-white metal belonging to Group 1 of the Periodic Table, which is the alkali metals group.
1. Sodium is highly reactive! The pure metal is kept under oil or kerosene because it spontaneously ignites in water. It's interesting to note, sodium metal also floats on water!
2. Room temperature sodium metal is soft enough that you can cut it with a butter knife.
3. Sodium is an essential element for animal nutrition. In humans, sodium is important for maintaining fluid balance in the cells and throughout the body. The electric potential maintained by sodium ions is critical for nerve function.
4. Sodium and it compounds are used for food preservation, cooling nuclear reactors, in sodium vapor lamps, to purify and refine other elements and compounds, and as a desiccant.
5. There is only one stable isotope of sodium, 23Na.
6. The symbol for sodium is Na, which comes from the Latin natrium or Arabic natrun or a similar-sounding Egyptian word, all referring to soda or sodium carbonate.
7. Sodium is an abundant element. It is found in the sun and many other stars. It is the 6th most abundant element on Earth, comprising about 2.6% of the earth's crust. It is the most abundant alkali metal.
8. Although it too reactive to occur in pure elemental form, it is found in many minerals, including halite, cryolite, soda niter, zeolite, amphibole, and sodalite. The most common sodium mineral is halite or sodium chloride salt.
9. Sodium first was commercially produced by by thermal reduction of sodium carbonate with carbon at 1100°C, in the Deville process. Pure sodium may be obtained by electrolysis of molten sodium chloride. It may be produced by by the thermal decomposition of sodium azide.
 Calcium is a metal. It readily oxidizes in air. Because it makes up such a large part of the skeleton, about one-third of the mass of human body comes from calcium, after water has been removed.
Calcium is a metal. It readily oxidizes in air. Because it makes up such a large part of the skeleton, about one-third of the mass of human body comes from calcium, after water has been removed.
Calcium is one of the elements you need in order to live, so it's worth knowing a little bit about it. Here are some quick facts about the element calcium. You can find more calcium facts on the calcium facts page.
1. Calcium isn't found free in nature, but it can be purified into a soft silvery-white alkaline earth metal.
2. Calcium is the 5th most abundant element in the Earth's crust, present at a level of about 3% in the oceans and soil.
3. The element is essential for animal and plant nutrition. Calcium participates in many biochemical reactions, including building skeletal systems and moderating muscle action.
4. Vitamin D is essential for calcium absorption by the human body. Vitamin D is converted to a hormone which causes intestinal proteins responsible for calcium absorption to be produced.
5. While calcium and its compounds are not considered to be toxic, ingesting too many calcium carbonate dietary supplements or antacids can cause milk-alkali syndrome, which is associated with hypercalcemia sometimes leading to fatal renal failure. Excessive consumption would be on the order of 10 g calcium carbonate/day, though symptoms have been reported upon ingesting as little as 2.5 g calcium carbonate daily.
6. Calcium is used for making cement, making cheese, removing nonmetallic impurities from alloys, and as a reduction agent in the preparation of other metals.
7. Pure calcium metal reacts vigorously and sometimes violently with water and acids.
8. The element name "calcium" comes from the Latin word "calcis" meaning "lime".
|
|
9. Calcium has been known since the 1st century, when the ancient Romans were known to make lime from calcium oxide.
10.Though calcium has been known for thousands of years, it was not purifed as an element until 1808 by Sir Humphrey Davy (England).
 Carbon is the basis for organic chemistry, as it occurs in all living organisms.
Carbon is the basis for organic chemistry, as it occurs in all living organisms.
2. Carbon is a nonmetal that can bond with itself and many otherchemical elements, forming nearly ten million compounds.
3. Elemental carbon can take the form of one of the hardest substances (diamond) or one of the softest (graphite).
4. Carbon is made in the interiors of stars, though it was not produced in the Big Bang.
5. Carbon compounds have limitless uses. In its elemental form, diamond is a gemstone and used for drilling/cutting; graphite is used in pencils, as a lubricant, and to protect against rust; while charcoal is used to remove toxins, tastes, and odors. The isotope Carbon-14 is used in radiocarbon dating.
6. Carbon has the highest melting/sublimation point of the elements. The melting point of diamond is ~3550°C, with the sublimation point of carbon around 3800°C.
7. Pure carbon exists free in nature and has been known since prehistoric time.
8. The origin of the name 'carbon' comes from the Latin word carbo, for charcoal. The German and French words for charoal are similar.
9. Pure carbon is considered non-toxic, although inhalation of fine particles, such as soot, can damage lung tissue.
10. Carbon is the fourth most abundant element in the universe (hydrogen, helium, and oxygen are found in higher amounts, by mass).

This is the glow given off by ionized nitrogen in a gas discharge tube. The purplish glow seen around lightning strikes is the color of the ionized nitrogen in air.
You breathe oxygen, yet air is mostly nitrogen. You need nitrogen to live and encounter it in the foods you eat and in many common chemicals. Here are some quick facts about this element. You can find detailed information about nitrogen on the nitrogen facts page.
1. Nitrogen is odorless, tasteless, and colorless.
2. Nitrogen gas (N2) makes up 78.1% of the volume of the Earth's air.
3. Nitrogen is a nonmetal.
4. Nitrogen gas is relatively inert, but soil bacteria can 'fix' nitrogen into a form that plants and animals can use to make amino acids and proteins.
5. The French chemist Antoine Laurent Lavoisier named nitrogen azote, meaning without life.
6. Nitrogen was sometimes referred to as 'burnt' or 'dephlogisticated' air, since air that no longer contains oxygen is almost all nitrogen. The other gases in air are present in much lower concentrations.
7. Nitrogen compounds are found in foods, fertilizers, poisons, and explosives. Your body is 3% nitrogen by weight.
8. Nitrogen is responsible for the orange-red, blue-green, blue-violet, and deep violet colors of the aurora.
9. One way to prepare nitrogen gas is by liquefaction and fractional distillation from the atmosphere.
10.Nitrogen has a valence of 3 or 5.
Types of Chemical Reactions
A chemical reaction is a process that is usually characterized by a chemical change in which the starting materials (reactants) are different from the products. Chemical reactions tend to involve the motion of electrons, leading to the formation and breaking of chemical bonds. There are several different types of chemical reactions and more than one way of classifying them. Here are some common reaction types. However, if you are asked to name the main 4, 5 or 6 types of chemical reactions, here is how they are categorized.
· Direct Combination or Synthesis Reaction
In a synthesis reaction two or more chemical species combine to form a more complex product.
A + B → AB
The combination of iron and sulfur to form iron (II) sulfide is an example of a synthesis reaction:
8 Fe + S8 → 8 FeS
Learn More About Synthesis Reactions
· Chemical Decomposition or Analysis Reaction
In a decomposition reaction a compound is broken into smaller chemical species.
|
|
AB → A + B
The electrolysis of water into oxygen and hydrogen gas is an example of a decomposition reaction:
2 H2O → 2 H2 + O2
· Single Displacement or Substitution Reaction
A substitution or single displacement reaction is characterized by one element being displaced from a compound by another element.
A + BC → AC + B
An example of a substitution reaction occurs when zinc combines with hydrochloric acid. The zinc replaces the hydrogen:
Zn + 2 HCl → ZnCl2 + H2
· Metathesis or Double Displacement Reaction
In a double displacement or metathesis reaction two compounds exchange bonds or ions in order to form different compounds.
AB + CD → AD + CB
An example of a double displacement reaction occurs between sodium chloride and silver nitrate to form sodium nitrate and silver chloride.
NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
· Acid-Base Reaction
An acid-base reaction is type of double displacement reaction that occurs between an acid and a base. The H+ ion in the acid reacts with the OH- ion in the base to form water and an ionic salt:
HA + BOH → H2O + BA
The reaction between hydrobromic acid (HBr) and sodium hydroxide is an example of an acid-base reaction:
HBr + NaOH → NaBr + H2O
· Oxidation-Reduction or Redox Reaction
In a redox reaction the oxidation numbers of atoms are changed. Redox reactions may involve the transfer of electrons between chemical species.
The reaction that occurs when In which I2 is reduced to I- and S2O32- (thiosulfate anion) is oxidized to S4O62- provides an example of a redox reaction:
2 S2O32−(aq) + I2(aq) → S4O62−(aq) + 2 I−(aq)
· Combustion
A combustion reaction is a type of redox reaction in which a combustible material combines with an oxidizer to form oxidized products and generate heat (exothermic reaction). Usually in a combustion reaction oxygen combines with another compound to form carbon dioxideand water. An example of a combustion reaction is the burning of naphthalene:
C10H8 + 12 O2 → 10 CO2 + 4 H2O
Learn More About Combustion Reactions
· Isomerization
In an isomerization reaction, the stuctural arrangement of a compound is changed but its net atomic composition remains the same.
· Hydrolysis Reaction
A hydrolysis reaction involves water. The general form for a hydrolysis reaction is:
X-(aq) + H2O(l) <--> HX(aq) + OH-(aq)
Basic Laws of chemistry
1. Law of mass conversation (M.V. Lomonosov 1748, A. Lavoisier 1789).
The mass of the substances entering into a reaction equal the mass of the substances formed as a result of the reaction.
Arranging of chemical equations
Include three stages:
1. Record formulas of substances: entered in the reaction (on the left) and products of reaction (on the right), having connected them on the sense by signs «+», «®»:HgO ®Hg + O2
2. Selection the coefficients for each substance so that amount of atoms of each element in left and right part of equation will be equally: 2HgO ® 2Hg + O2
3. Checking a number of atoms of each element in left and right parts of equation.
2. Law of constant composition. For the first time has formulated by
G. Prust (1808).
All individual chemical substances have constant quality and quantity composition and definite chemical structure and does not depend on how this substance was prepared.
From the law of constant composition follows that at complex substance formation the elements combine with each other in definite mass proportions.
|
|
example
CuS- copper sulphide. m (Cu): m (S) = Ar (Cu): Ar (S) = 64: 32 = 2: 1
To get copper sulphide (CuS) it is necessary to mix up the powders of copper and sulphur in mass relations 2:1.
If taken amounts of source substances do not correspond their correlation in the chemical formula of compound one of them stay in the excess.
3. Law of multiple proportions (D. Dalton, 1803)
If two elements form several chemical compounds with each other, then the masses of one of the elements corresponding to the same mass of the other element in these compounds are in a simple integral proportion.
N2O N2O3 N2O5
A number of oxygen atoms in molecules of such compounds corresponding to the two nitrogen atoms are in a proportion 1:3:5.
4. Law of combining volumes (Gay-Lussac, 1808).
When gases react, the volumes consumed and produced, measured at the same temperature and pressure, are in ratios of small whole numbers. Volumes of reacting gases bear a simple whole number ratio.
Consequence. Stoichiometric coefficients in equations of chemical reactions for molecules of gaseous substances shows in which volume combinations gaseous substances are got or react.
Avogadro law (1811).
Equal volumes of all gases at the same conditions (temperature, pressure) contain the same number of molecules.
This law truth for gaseous substances only.
Consequences:
1. On mole of any substance in the gaseous state occupies the same volume at the same temperature and pressure.
2. One mole of any gas in standard conditions (0°C = 273°K, 1 atm = 101.3 kPa) occupies a volume of 22.4 litres.
General gas law
General gas law is association of three independent private gas laws: Gay-Lussac’s, Charle’s, Boyl’s- Mariott’s, equation which possible write like this:
P1V1/T1=P2V2/ T2
Conversely, from general gas law under P= const. (P1=P2), possible to get:
V1/T1= V2/T2 (Gay-Lussac’s law)
On the T=const. (T1=T2):
P1V1= P2V2 (Boyl’s-Mariott’s law).
On the V=const.
P1/T1=P2/T2 (Charle’s law).
Clapeyron’s - Mendeleyev’s equation
If write general gas law for any mass of any gas than would be Clapeyron’s - Mendeleyev’s equation:
PV= 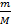 RT
RT
where, m - gas mass; M - molecular mass; p - pressure; V - volume; T - absolute temperature (°K); R - molar gas constant (8.314 J/(mol·K) or 0.082 l atm/(mol·K)).
For given mass of concrete gas the ratio m/M is a constant, therefore general gas law is obtained from Clapeyron’s - Mendeleyev’s equation.
Control questions
1. What das the law of mass proportion state?
2. What das the law of multiple proportions state?
3. What does Avogadro's principle state?
4. What is the chemical reaction?
5. What is a compound molecule?
6. Use the idel-gas-law equation to calculate the unknown quantity in each of the fillowing sets of measurements. You will need to convert Celsius temperatures to Kelvin temperatures and volume units to litres.
| № | P | V | n | T |
| 6 | 1.09 atm | ? L | 0.0881 mol | 302 K |
| 7 | 94.9 kPa | 0.0350 L | ? mol | 550C |
| 8 | ? kPa | 15.7 L | 0.815 mol | -200C |
| 9 | 0.500 atm | 629 ml | 0.0337 mol | ? K |
| 10 | 0.950 atm | ? L | 0.0818 mol | 190C |
| 11 | 1.07 atm | ? L | 0.0962 mol | 352 K |
| 12 | 81.9 kPa | 0.0260 L | ? mol | 1000C |
| 13 | ? kPa | 25.7 L | 0.752 mol | 620C |
| 14 | 0.600 atm | 723 ml | 0.0336 mol | ? K |
| 15 | 0.960 atm | ? L | 0.0825 mol | 260C |
16. Calculate the equivalent weights for the following substances:
| № | substances |
| 16 | MgO, HClO4, NaH2PO4, Al2O3, HNO3, NH4Cl |
| 17 | MnO2, H2SO3, Ni(OH)Cl, SnO2, Fe(OH)2, CuSO4 |
| 18 | NO, H3PO4, Hg(NO3)2, Mn2O3, H2CO3, Ca(H2PO4)2 |
| 19 | Fe2O3, HBr, KHSO4, NO2, Fe(OH)3, Al(HSO4)3 |
| 20 | CuO, H3PO3, MgCO3, Cr2O3, HI, Fe(OH)SO4 |
| 21 | PbO2, Sn(OH)2, Zn(OH)Cl, N2O5, H3AlO3, SnCl2 |
| 22 | CO2, HNO2, Ca3(PO4)2, CrO3, H2S, AuCl3 |
| 23 | SO3, KHS, Pb(NO3)2, P2O5, HMnO4, SrCO3 |
| 24 | PbO, H2CrO4, NaHSO3, SO2, K2HPO4, Ag2SO4 |
| 25 | FeO, H2SiO3, Na2HPO3, SeO2, H2SO3, KAlO2 |
Control questions
1. By what property did Moseley suggest that the periodic table be arranged?
2. What is the periodic law?
3. What is a period? How many are there in the periodic table?
4. What is a group (also called a family)? How many are there in the periodic table?
5. State the number of valence electrons in an atom of:
a. sulfur b. calcium c. chlorine d. arsenic
6. Give the names and chemical symbols for the elements that correspond to these atomic numbers:
a. 10 b. 18 c. 36 d. 90
|
|
7. List, by number, both the period and group of each of these elements.
Symbol Period Group
a. beryllium Be
b. iron Fe
c. lead Pb
8. Which of the following pairs of elements belong to the same period?
a. Na and Cl b. Na and Li c. Na and Cu d. Na and Ne
9. Which of the following pairs of elements belong to the same group?
a. H and He b. Li and Be c. C and Pb d. Ga and Ge
10. How does an element’s period number relate to the number of the energy level of its valence electrons?
11. What is the heaviest noble gas? What is the heaviest alkaline earth metal?
12. What is the name given to the group of elements that have the following valence shell electron configurations?
a. s2 b. s2p6 c. s2p5 d. s1
13. List the three lightest members of the noble gases.
14. List all of the alkali metals.
15. Why do all the members of a group have similar properties?
16. What do we mean by the “atomic radius?”
17. Within a group, what happens to the atomic radius as you go down the column?
18. How are the shielding effect and the size of the atomic radius related?
19. What is ionization energy?
20. Which of these elements has the highest first ionization energy: Sn, As, or S?
21. When an atom becomes an anion, what happens to its radius?
22. When an atom becomes a cation, what happens to its radius?
23. For each of the following pairs, circle the atom or ion having the larger radius.
a. S or O c. Na1+ or K1+ e. S2– or O
b. Ca or Ca2+ d. Na or K f. F or F1–
24. For each of the following pairs, identify the smaller ion.
a. K1+ or Ca2+ c. C4+ or C4– e. O2– or F1–
b. F1– or Cl1– d. S2– or F1– f. Fe2+ or Fe3+
25. Where, generally, are the metals located on the periodic table?
26. Where, generally, are the nonmetals located on the periodic table?
A. List some properties of metals.
B. List some properties of nonmetals.
C. What kinds of properties do metalloids have?
27. What is electronegativity?
28. Who determined the scale of electronegativity most often used today?
29. List the following atoms in order of increasing electronegativity:
O, Al, Ca
30. List the following atoms in order of decreasing electronegativity:
Cl, K, Cu
31. Arrange these elements according to decreasing atomic size:
Na, C, Sr, Cu, Fr
32. Arrange these elements according to increasing negative E. A.:
Ba, F, Si, Ca, O
33. Arrange these elements according to increasing metallic character:
Li, S, Ag, Cs, Ge
34. Which reaction do you expect to have the greater cell potential?
A) 2Na(s) + Cl2(g)→ 2NaCl(s) or 2Cs(s) +Cl2(g) → 2RbCl(s)
B) 2Na(s) + Cl2(g)→ 2NaCl(s) or Be(s) + Cl2(g) → BeCl2(s)
35. Which equation do you expect to occur?
A) I2(s) + 2Br (aq) → Br2(l) + 2I(aq)
B) Cl2(g) + 2I (aq) → I2 (s) + 2Cl(aq)
Chemical Bonds & Compounds Chemistry Quiz
This ten question multiple-choice quiz will test your understanding of the types of chemical bonds, how electrons are transferred between elements in a compound, and how compounds form. You may wish to review atoms and ions and chemical formulas before you begin. Refer to the periodic table for information about the elements, as needed.
Question: What Is the Difference Between an Ionic and Covalent Chemical
Bond?
Answer: A molecule or compound is made when two or more atoms form a chemical bond, linking them together. Molecule refers to two or more atoms which have chemically combined to form a single species.
Examples of molecules include water H2O, oxygen, gas, O2
A covalent bond is a chemical link between two atoms in which electrons are shared between them.
Examples: There is a covalent bond between the oxygen and each hydrogen in a water molecule (H2O). Each of the covalent bonds contains two electrons - one from a hydrogen atom and one from the oxygen atom. Both atoms share the electrons.
The two types of bonds are ionic bonds and covalent bonds. In an ionic bond, the atoms are bound together by the attraction between oppositely-charged ions. For example, sodium and chloride form an ionic bond, to make NaCl, or table salt. In a covalent bond, the atoms are bound by shared electrons. If the electron is shared equally between the atoms forming a covalent bond, then the bond is said to be nonpolar. Usually, an electron is more attracted to one atom than to another, forming a polar covalent bond. For example, the atoms in water, H2O, are held together by polar covalent bonds. Do you understand? Test your comprehension with this quiz.
Acids produce H+ ions in aqueous solutions, bases produce OH- ions in aqueous solutions. Water required, so only allows for aqueous solutions. Only protic acids are allowed; required to produce hydrogen ions. Only hydroxide bases are allowed.
Binary Acids
A binary compound consists of two elements. Binary acids have the prefix hydro in front of the full name of the nonmetallic element. They have the ending -ic. Examples include hydrochloric and hydrofluoric acid.
Hydrofluoric Acid - HF
Hydrochloric Acid - HCl
Hydrobromic Acid - HBr
Hydroiodic Acid - HI
Hydrosulfuric Acid - H2S
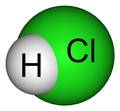
Hydrochloric Acid
Ternary Acids
Ternary acids commonly contain hydrogen, a nonmetal, and oxygen. The name of the most common form of the acid consists of the nonmetal root name with the -ic ending, The acid containing one less oxygen atom than the most common form is designated by the -ous ending. An acid containing one less oxygen atom than the -ous acid has the prefix hypo- and the -ous ending. The acid containing one more oxygen than the most common acid has the per- prefix and the -ic ending.
Nitric Acid - HNO3 Nitrous Acid - HNO2
Hypochlorous Acid – HClO Chlorous Acid - HClO2
Chloric Acid - HClO3 Perchloric Acid - HClO4
Sulfuric Acid - H2SO4 Sulfurous Acid - H2SO3
Phosphoric Acid - H3PO4 Phosphorous Acid - H3PO3
Carbonic Acid - H2CO3 Acetic Acid - HC2H3O2
Oxalic Acid - H2C2O4 Boric Acid - H3BO3
Silicic Acid - H2SiO3
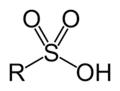
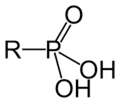
Sulfurous Acid Phosphoric Acid
These are the strong acids. What makes them 'strong' is that they completely dissociate into their ions (H+ and an anion) when they are mixed with water. Any other acid is a weak acid. There are only six strong acids, so you might want to commit the list of strong acids to memory.
HCl - hydrochloric acid
HNO3 - nitric acid
H2SO4 - sulfuric acid
HBr - hydrobromic acid
HI - hydroiodic acid (also known as hydriodic acid)
HClO4 - perchloric acid
As the strong acids become more concentrated, they may be unable to fully dissociate. The rule of thumb is that a strong acid is 100% dissociated in solutions of 1.0 M or less.
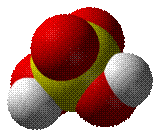
Sulfuric acid is one of the strong acids.
Bases
Sodium Hydroxide - NaOH
Potassium Hydroxide - KOH
Ammonium Hydroxide - NH4OH
Calcium Hydroxide - Ca(OH)2
Magnesium Hydroxide - Mg(OH)2
Barium Hydroxide - Ba(OH)2
Aluminum Hydroxide - Al(OH)3
Ferrous Hydroxide or Iron (II) Hydroxide - Fe(OH)2
Ferric Hydroxide or Iron (III) Hydroxide - Fe(OH)3
Zinc Hydroxide - Zn(OH)2
Lithium Hydroxide – LiOH
|
|
|
Типы сооружений для обработки осадков: Септиками называются сооружения, в которых одновременно происходят осветление сточной жидкости...

Таксономические единицы (категории) растений: Каждая система классификации состоит из определённых соподчиненных друг другу...
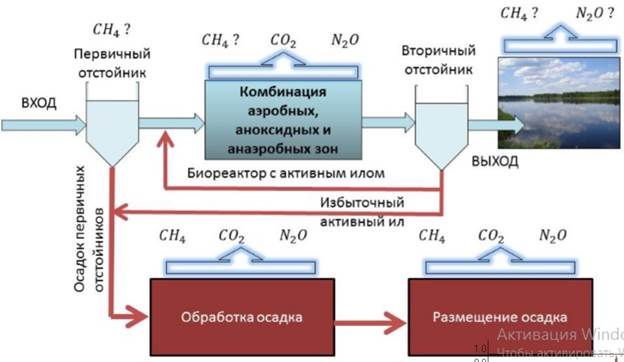
Эмиссия газов от очистных сооружений канализации: В последние годы внимание мирового сообщества сосредоточено на экологических проблемах...
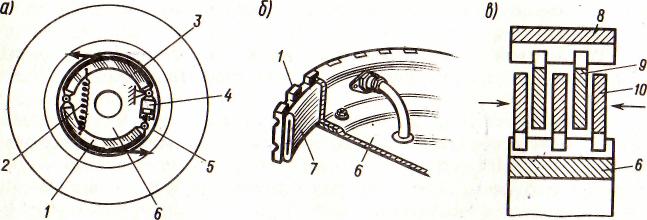
Автоматическое растормаживание колес: Тормозные устройства колес предназначены для уменьшения длины пробега и улучшения маневрирования ВС при...
© cyberpedia.su 2017-2024 - Не является автором материалов. Исключительное право сохранено за автором текста.
Если вы не хотите, чтобы данный материал был у нас на сайте, перейдите по ссылке: Нарушение авторских прав. Мы поможем в написании вашей работы!