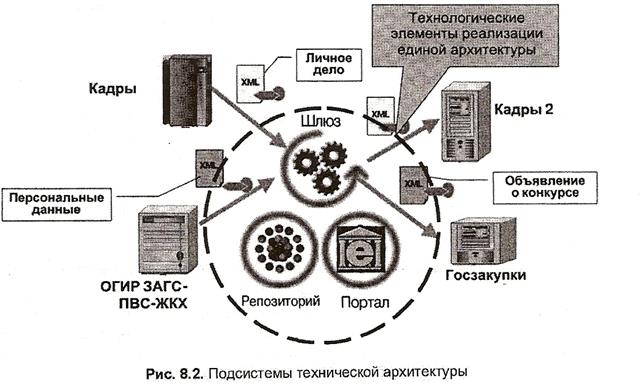
Elementary particles possess an intrinsic quantum mechanical property known as spin. This is analogous to the angular momentum of an object that is spinning around its center of mass, although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced Planck constant (ħ), with electrons, protons and neutrons all having spin ½ ħ, or "spin-½". In an atom, electrons in motion around the nucleus possess orbital angular momentum in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.
In ferromagnetic elements such as iron, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a process known as an exchange interaction. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. Paramagnetic materials have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field.
The nucleus of an atom can also have a net spin. Normally these nuclei are aligned in random directions because of thermal equilibrium. However, for certain elements (such as xenon-129) it is possible to polarize a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called hyperpolarization. This has important applications in magnetic resonance imaging.
Energy levels
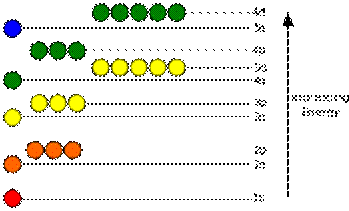
These electron's energy levels (not to scale) are sufficient for ground states of atoms up to cadmium (5s2 4d10) inclusively. Do not forget that even the top of the diagram is lower than an unbound electron state.
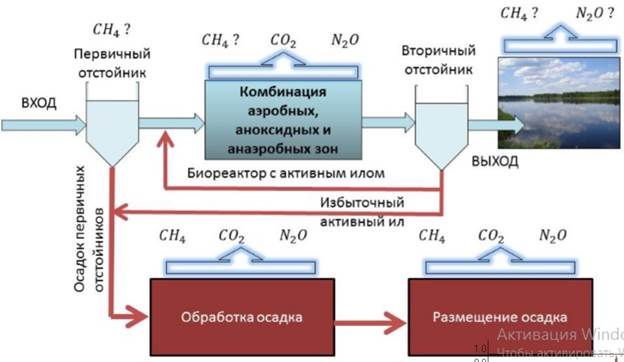
The potential energy of an electron in an atom is negative, its dependence of its positionreaches the minimum (the most absolute value) inside the nucleus, and vanishes when thedistance from the nucleus goes to infinity, roughly in an inverse proportion to the distance. In the quantum-mechanical model, a bound electron can only occupy a set of states centered on the nucleus, and each state corresponds to a specific energy level; see time-independent Schrödinger equation for theoretical explanation. An energy level can be measured by theamount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, while an electron transition to a higher level results in an excited state. The electron's energy raises when n increases because the (average) distance to the nucleus increases. Dependence of the energy on ℓ is caused not by electrostatic potential of the nucleus, but by interaction between electrons.
If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.
What is the Periodic Table?
Dmitri Mendeleev was the first scientist to create a periodic table of the elements similar to the one we use today. You can see Mendeleev's original table (1869). This table showed that when the elements were ordered by increasing atomic weight, a pattern appeared where properties of the elements repeated periodically. This periodic table is a chart that groups the elements according to their similar properties.
Mendeleev's Table
Compare the modern periodic table with Mendeleev's table. What do you notice? Mendeleev's table didn't have very many elements, did it? He had question marks and spaces between elements, where he predicted undiscovered elements would fit.
Discovering Elements
Remember changing the number of protons changes the atomic number, which is the number of the element. When you look at the modern periodic table, do you see any skipped atomic numbers that would be undiscovered elements? New elements today aren't discovered. They are made. You can still use the periodic table to predict the properties of these new elements.
Properties and Trends
The periodic table helps predict some properties of the elements compared to each other. Atom size decreases as you move from left to right across the table and increases as you move down a column. Energy required to remove an electron from an atom increases as you move from left to right and decreases as you move down a column. The ability to form a chemical bond increases as you move from left to right and decreases as you move down a column.
Today's Table
The most important difference between Mendeleev's table and today's table is the modern table is organized by increasing atomic number, not increasing atomic weight. Why was the table changed? In 1914, Henry Moseley learned you could experimentally determine the atomic numbers of elements. Before that, atomic numbers were just the order of elements based on increasing atomic weight. Once atomic numbers had significance, the periodic table was reorganized.
It's easier to navigate the periodic table and write chemical equations and formulae once you know the symbols for the elements. However, sometimes it's easy to confuse symbols of elements with similar names. Other elements have symbols that don't seem to relate to their names at all! For these elements, the symbol usually refers to an older element name that isn't used any more. Here's an alphabetical list of element symbols with the corresponding element name. Keep in mind that the names for the elements (and their symbols) may be different in languages other than English.
These are the element groups found in the periodic table of the elements. There are links to lists of elements within each group.