
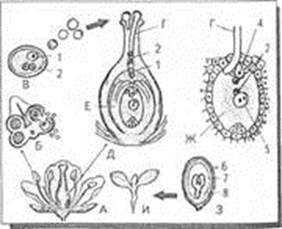
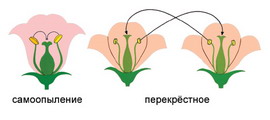
Двойное оплодотворение у цветковых растений: Оплодотворение - это процесс слияния мужской и женской половых клеток с образованием зиготы...
Семя – орган полового размножения и расселения растений: наружи у семян имеется плотный покров – кожура...

Двойное оплодотворение у цветковых растений: Оплодотворение - это процесс слияния мужской и женской половых клеток с образованием зиготы...

Семя – орган полового размножения и расселения растений: наружи у семян имеется плотный покров – кожура...
Топ:
Методика измерений сопротивления растеканию тока анодного заземления: Анодный заземлитель (анод) – проводник, погруженный в электролитическую среду (грунт, раствор электролита) и подключенный к положительному...
Особенности труда и отдыха в условиях низких температур: К работам при низких температурах на открытом воздухе и в не отапливаемых помещениях допускаются лица не моложе 18 лет, прошедшие...
Устройство и оснащение процедурного кабинета: Решающая роль в обеспечении правильного лечения пациентов отводится процедурной медсестре...
Интересное:
Распространение рака на другие отдаленные от желудка органы: Характерных симптомов рака желудка не существует. Выраженные симптомы появляются, когда опухоль...
Берегоукрепление оползневых склонов: На прибрежных склонах основной причиной развития оползневых процессов является подмыв водами рек естественных склонов...
Подходы к решению темы фильма: Существует три основных типа исторического фильма, имеющих между собой много общего...
Дисциплины:
|
из
5.00
|
Заказать работу |
|
|
|
|
Solution preparation with the set mass fraction of the dissolved substance from a hinge plate of strong substance.
Work purpose: To learn to prepare solutions different concentration, to learn to work with measured ware.
Devices and Reactants: test tubes, areometer, thermometer, measured cylinder, CaCl2, the distilled water.
Receive at the teacher a task for preparation of solution of strong substance in water.
Table 1
| Dissolved substance | the Volume of solution, V | Mass fraction of the dissolved substance, ω |
Dissolved substance (gets out the teacher from among the strong substances given in table 1)
Carry out necessary calculations and define:
- mass of solution,
- the mass of the dissolved substance,
- mass of solvent,
- solvent volume.
The mass of solution we find, using a formula:
msolution = Vsolution ∙ ρsolution,
where
msolution – the mass of solution, [g]
The V solution – the volume of solution, [cm3]
Ρ solution – density of solution, [g/cm3]
For finding of value of density of solution on a preset value of a mass fraction ωтеор. use tabular data, see table 1.
If the preset value of a mass fraction of the dissolved substance doesn't coincide with the given tabular values, for finding of density of solution of the set concentration of r teor the interpolation method is used.
For example:
It is required to find value of density of solution of chloride of calcium (CaCl2) with a mass fraction of the dissolved substance ω (CaCl2) = 10,2%.
Values of density of solution with a mass fraction of 10,2% in the table aren't present. Therefore we will write out the next to demanded tabular values:
| ω (CaCl2), % | ρsolution CaCl2, g/cm3 |
| 10 | 1,084 |
| 12 | 1,102 |
Change of concentration of solution of this substance = 2% leads on 12-10 to change of density of the solution, equal 1,102-1,084 = 0,018 g/cm3.
The value of a mass fraction of solution set to us exceeds the next smaller tabular value on 10,2 – 10 = 0,2%.
Let's make a proportion and we will find as far as solution density with ω = 10,2%, in comparison with solution density with ω = 10% will increase:
2% - 0,018 g/cm3
0,2% - x. x = 0,0018 g/cm3.
Thus, density of solution of chloride of calcium with
ω = 10,2% will be equal:
ρ (10,2%) = ρ (10%) + x = 1,084 + 0,0018 = 1,0858 ≈ 1,086 g/cm3.
The mass of the dissolved substance we determine by a formula:
m = msolution ∙ ω, where
ω – a mass fraction of the dissolved substance in the solution, expressed in unit shares.
The mass of solvent we will determine as a difference between the mass of solution and the dissolved substance.
msolvent = msolution – mdissolved substance
As it is more convenient to liquid not to weigh, and to measure for them a certain volume, we will find the volume which will be occupied by the found mass of solvent.
|
|
For finding of volume of solvent, we use a formula: V= m/ r
where
m – the mass of solvent, [g]
V – the volume of solvent, [cm3]
r solvent – density of solvent, [g/cm3]
Let's remind that water density at 200 C ρ (H2O) = 1 g/cm3
Give calculations and write down all settlement data.
Table 2. Data for preparation of solution of the set quantitative structure
| Size | Designation | Value | Dimension |
| Density of solution of the set quantitative structure | Ρ solution theoretically | [g/cm3] | |
| mass of solution is | m solutions | [g] | |
| The mass of the dissolved substance | m dissolved substance | [g] | |
| The mass of solvent | m solvents | [g] | |
| The volume of solvent | V solvents | [cm3] |
The dissolved substance, the found weight, weigh on scales. The necessary volume of the distilled water measure the measured cylinder.
Prepare solution in a chemical glass, mixing a glass stick. The thermometer take solution temperature. If temperature differs from 20 °C, solution needs to be heated or cooled.
Pour solution in the 50 cm3 cylinder, carefully lower in it the areometer and by an areometer scale (on the bottom meniscus of liquid) determine density of the prepared solution.
It is necessary to remember that during determination of density of solution, the areometer shouldn't concern cylinder walls. After each definition the areometer wash, wipe filter paper and carefully place in the corresponding nest of a support.
On the measured density of rexp using, in case of need, an interpolation method according to tab. 1 determine value of a mass fraction of the dissolved substance in the prepared solution (ωexp.).
Compare the found value (ωexp.) to a preset value of a mass fraction (ωteor.) also define value of a relative error of experiment h:
h = (ωexp·. ωteor./ ωteor.)·100
Give calculations and write down in tab. 3 the obtained experimental and settlement data.
Table 3. Results of experiment on preparation of solution of the set quantitative structure.
| Size | Designation | Value | Dimension |
| The measured density of the prepared solution | r solution of the exp | [g/cm3] | |
| Mass fraction of the dissolved substance in the prepared solution | ω exp. | - | |
| Relative error of experiment | h | - |
For solution with the set mass fraction express quantitative structure in other ways. For this purpose calculate values of mass, painting and normal concentration and a molyality of this solution.
Laboratory work 5
Solutions of electrolytes
Operation purpose: To study reaction courses with participation of electrolytes.
Devices and reactants: NH4Cl, NaOH, CH3COONa, drop tablet, universal indicator, acetic acid, glass stick.
Experience 1. Definition of the direction of course of reactions with participation of electrolytes.
Reactions with participation of electrolytes proceed in the direction of formation of weak electrolytes, allocation of gases and loss of a precipitation.
Bring in a cell of a drop tablet a small amount of crystal chloride of NH4Cl ammonium and add 2-3 drops of 1 N of solution caustic натра NaOH. Mix cell contents a glass rake, determine by a smell, what gas is emitted. Write down the reaction equation in a molecular and ionic look. Explain the reason of liberation of gas.
|
|
Bring in a cell of a drop tablet a small amount of crystal acetate of CH3COONa sodium and add 2-3 drops of 1 N of solution of hydrochloric HCl acid. Mix cell contents, define, to what connection there corresponds a new smell. Write down the reaction equation in a molecular and ionic look.
Draw a conclusion on the direction of course of the studied reactions, using data on constants of dissociation of the corresponding electrolytes.
Kb (NH4OH)=1,77 ·10-5, Kа (СН3СOONа)=1,86·10-5
Experience 2. Shift of balance of dissociation of weak electrolyte.
According to Le-Shatelye's principle balance of dissociation of weak electrolyte can be displaced, adding in solution the connections containing the ions of the same name.
Bring 2-3 drops of 0,1 N of solution of acetic acid in a cell of a drop tablet. By means of the universal indicator, as well as in experience 4, estimate value рН solution. Add a small amount of crystal CH3COONa and mix cell contents a glass rake. Again using the universal indicator, estimate value рН the received solution. Explain the reasons of change of coloring of the indicator, write the equations of processes.
Repeat actions of item 1 using 0,1 N NH4OH solution and crystal chloride of NH4Cl ammonium. (It is possible to use a cell and data of the previous experience.) Using expressions of constants of dissociation of acetic acid and ammonium hydroxide, explain the occurred changes on the basis of Le-Shatelye's principle.
Kа (СН3СOONа)=1,86·10-5; Kb(NH4OH)=1,77·10-5
Laboratory work 6
|
|
|
Наброски и зарисовки растений, плодов, цветов: Освоить конструктивное построение структуры дерева через зарисовки отдельных деревьев, группы деревьев...
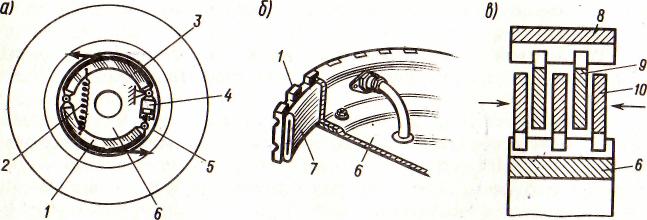
Автоматическое растормаживание колес: Тормозные устройства колес предназначены для уменьшения длины пробега и улучшения маневрирования ВС при...
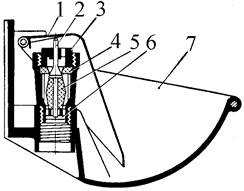
Индивидуальные и групповые автопоилки: для животных. Схемы и конструкции...

Двойное оплодотворение у цветковых растений: Оплодотворение - это процесс слияния мужской и женской половых клеток с образованием зиготы...
© cyberpedia.su 2017-2024 - Не является автором материалов. Исключительное право сохранено за автором текста.
Если вы не хотите, чтобы данный материал был у нас на сайте, перейдите по ссылке: Нарушение авторских прав. Мы поможем в написании вашей работы!