Inorganic polymers are polymers with a skeletal structure that does not include carbon atoms in the backbone.[1]Polymers containing inorganic and organic components are sometimes called hybrid polymers,[2] and most so-called inorganic polymers are hybrid polymers. [3]One of the best known examples is polydimethylsiloxane, otherwise known commonly as silicone rubber. Inorganic polymers offer some properties not found in organic materials including low temperature flexibility, electrical conductivity, and nonflammability.[4] The term inorganic polymer refers generally to one-dimensional polymers, rather than to heavily crosslinked materials such as silicate minerals. Inorganic polymers with tunable or responsive properties are sometimes called smart inorganic polymers. A special class of inorganic polymers are geopolymers, which may be anthropogenic or naturally occurring.
Borazine is a colourless liquid with an aromatic smell. In water it hydrolyzes to boric acid, ammonia, and hydrogen. Borazine, with a standard enthalpy change of formation ΔHf of −531 kJ/mol, is thermally very stable.
Describe the features of the physico-chemical properties of inorganic polymers based on phosphorus
Inorganic polymers are polymers with a skeletal structure that does not include carbon atoms in the backbone.[1]Polymers containing inorganic and organic components are sometimes called hybrid polymers,[2] and most so-called inorganic polymers are hybrid polymers. [3]One of the best known examples is polydimethylsiloxane, otherwise known commonly as silicone rubber. Inorganic polymers offer some properties not found in organic materials including low temperature flexibility, electrical conductivity, and nonflammability.[4] The term inorganic polymer refers generally to one-dimensional polymers, rather than to heavily crosslinked materials such as silicate minerals. Inorganic polymers with tunable or responsive properties are sometimes called smart inorganic polymers. A special class of inorganic polymers are geopolymers, which may be anthropogenic or naturally occurring.
The linear high polymers have the geometry shown in the picture. More than 700 different macromolecules that correspond to this structure are known with different side groupsor combinations of different side groups. In these polymers the properties are defined by the high flexibility of the backbone. Other potentially attractive properties include radiation resistance, high refractive index, ultraviolet and visible transparency, and its fire resistance. The side groups exert an equal or even greater influence on the properties since they impart properties such as hydrophobicity, hydrophilicity, color, useful biological properties such as bioerodibility, or ion transport properties to the polymers. Representativeexamplesofthesepolymersareshownbelow. 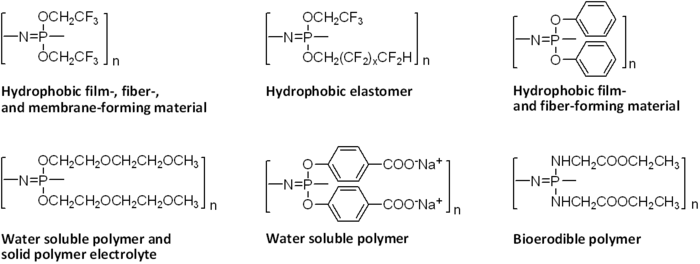
Describe the features of the physico-chemical properties of inorganic polymers based on alkali metals.25 Describe the features of the physico-chemical properties of inorganic polymers based on alkaline earth metals
The metal in main-group organometallic compounds can be any of the elements in the s-block (i.e., groups 1 and 2) or any of the heavier elements in groups 13 through 15. (Groups 1318 constitute the p block.) The elements at the borderline between the d block and pblocknamely, zinc, cadmium, and mercurywill be discussed along with the p-block organometallics because of the similarity of their organometallic chemistry.
The stability and reactivity of organometallic compounds
The stability and reactivity of organometallic compounds are associated with the nature of the organic ligands and the metal to which they are attached. In each of the main groups of the periodic table (groups 1, 2, and 1315), the thermal stability of a given type of organometallic compound generally decreases from the lightest to the heaviest element in a group.
This trend in stability is a consequence in part of the decrease in ondstrenth on oin don ithin a rop he trend does not hold for the d-block elements (groups 3 here ond strengths and stability often increase going down a group.
FORMATION OF ALKYLLITHIUM AND GRIGNARD REAGENTS
The highly active metals combine with a halogen-substituted hydrocarbon to produce simple organometallic compounds. For example, methyllithium, an important reagent in organic synthesis, is produced commercially. With other active metals, such as magnesium, aluminum, and zinc, the reaction generally yields the organometallic halide. A common reaction of this type is the synthesis of a Grignard reagent, an alkylmagnesium halide that finds wide use in organic synthesis (the s indicates that the metal is in solid form)
The synthesis of organometallic compounds by double displacement involves organometallic (MR) and binary halide (EX, where E is a metal or metalloid and X is a halogen) starting materials. It provides a convenient synthetic procedure that is widely used in the laboratory and to a lesser extent on a commercial scale. As the following examples illustrate, the organic group on the more active metal is transferred to the less active metal or metalloid. In this context the most common highly active metals are lithium, aluminum, and magnesium.
DOUBLE DISPLACEMENT
Double displacements involving the same central element are often referred to as redistribution reactions. A commercially important example is the redistribution of silicon tetrachloride and tetramethylsilicon (also known as tetramethylsilane) at elevated temperatures.
The products from this reaction can be separated by distillation. This reaction is performed industrially where (CH3)2SiCl2 is removed from the equilibrating mixture and then hydrolyzed to produce the intermediates for silicone polymers, which have the form in
REDISTRIBUTION
The addition of a metal hydride to a multiple bond is called hydrometallation, and it leads to the formation of a metal-carbon ond In the hydroboration and hydrosilation of an unsymmetrical alkene, the boron or siliconbinds to the carbon atom that has less-bulky substituents, and the smaller hydrogen atom binds to the carbon atom that has bulky substituents(CH3)2C. Hydroboration was discovered and developed in the United States by Herbert C. Brown, who shared the Nobel Prize for Chemistry in 1979 for this research. Both hydroboration and hydrosilation are widely used in the synthesis of complex organic molecules. In these applications the oriond is enerally cleaved in a subsequent step to produce a product that is free of boron or silicon.
HYDROMETALLATION
All organometallic compounds are potential reducing agents, and those of the electropositive elements are very strong reducing agents because the metal gives upelectrons to the caronresltin in a polar ondith a partial positive charge on the metal and a negative charge on the carbon. Organometallic compounds of highly electropositive elements such as lithium, sodium, and aluminum ignite spontaneously and sometimes explode on contact with air or other oxidizing agents. The useful organometallic reagents Li(CH3), Zn(CH3)2, B(CH3)3, and Al2(CH3)6 are spontaneously flammable in air (pyrophoric). Accordingly, techniques have been developed to handle these and other pyrophoric compounds. Glass reaction vessels sealed from the atmosphere and purged with nitrogen gas are commonly used for handling airsensitive organometallic compounds in the laboratory. Large quantities of pyrophoric compounds such as Al2(C2H5)6 are routinely handled with ease in the chemical industry by using closed metal reactors for the production of these and other much less reactive compounds. Organometallic compounds have reduced reactivity when the metallic component is not highly electropositive and when the metal is completely surrounded by attached groups. For example, elevated temperatures are required to initiate combustion with Si(CH3)4 and Sn(CH3)4, and at room temperature they can be handled in air.
REDUCTION
This is a common organometallic reaction in which a hydrogen atom on a carbon atom that is one position removed from the metal the position transfers to the metal ith the lieration of an alkene. The following example shows the formation of ethylene. This reaction is the reverse of the addition of an ond to an alkene, and under some conditions significant equilibrium concentrations of both reactants and products are observed. The -hydrogen elimination reaction is thought to proceed through the transition state, and as might be expected from this reaction mechanism, compounds with an accessible central metal atom tend to ndero -hydrogen elimination readily. For example, it occurs with trialkylaluminum compounds but not with tetraalkylsilicon compounds, in which reaction of the silicon atom is hindered by the bulky substituents.