
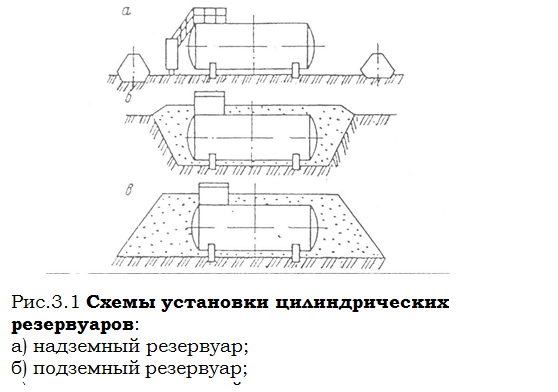
История развития хранилищ для нефти: Первые склады нефти появились в XVII веке. Они представляли собой землянные ямы-амбара глубиной 4…5 м...
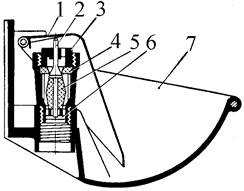
Индивидуальные и групповые автопоилки: для животных. Схемы и конструкции...

История развития хранилищ для нефти: Первые склады нефти появились в XVII веке. Они представляли собой землянные ямы-амбара глубиной 4…5 м...

Индивидуальные и групповые автопоилки: для животных. Схемы и конструкции...
Топ:
Методика измерений сопротивления растеканию тока анодного заземления: Анодный заземлитель (анод) – проводник, погруженный в электролитическую среду (грунт, раствор электролита) и подключенный к положительному...
Теоретическая значимость работы: Описание теоретической значимости (ценности) результатов исследования должно присутствовать во введении...
Марксистская теория происхождения государства: По мнению Маркса и Энгельса, в основе развития общества, происходящих в нем изменений лежит...
Интересное:
Влияние предпринимательской среды на эффективное функционирование предприятия: Предпринимательская среда – это совокупность внешних и внутренних факторов, оказывающих влияние на функционирование фирмы...
Распространение рака на другие отдаленные от желудка органы: Характерных симптомов рака желудка не существует. Выраженные симптомы появляются, когда опухоль...
Отражение на счетах бухгалтерского учета процесса приобретения: Процесс заготовления представляет систему экономических событий, включающих приобретение организацией у поставщиков сырья...
Дисциплины:
|
из
5.00
|
Заказать работу |
|
|
|
|
Useful information:
At STP: pressure = 1 atm = 700 mmHg, temperature = 0 °C = 273 K
At STP: 1 mole of gas occupies 22.4 L
R = ideal gas constant = 0.0821 L·atm/mol·K = 8.3145 J/mol·K
1 A balloon contains 4 moles of an ideal gas with a volume of 5.0 L.
If an additional 8 moles of the gas is added at constant pressure and temperature, what will be the final volume of the balloon?
2 What is the density (in g/L) of a gas with a molar mass of 60 g/mol at 0.75 atm and 27 °C?
A mixture of helium and neon gases is held in a container at 1.2 atmospheres. If the mixture contains twice as many helium atoms as neon atoms, what is the partial pressure of helium?
4 4 moles of nitrogen gas are confined to a 6.0 L vessel at 177 °C and 12.0 atm. If the vessel is allowed to expand isothermically to 36.0 L, what would be the final pressure?
5 A 9.0 L volume of chlorine gas is heated from 27 °C to 127 °C at constant pressure. What is the final volume?
6 The temperature of a sample of an ideal gas in a sealed 5.0 L container is raised from 27 °C to 77 °C. If the initial pressure of the gas was 3.0 atm, what is the final pressure?
7 A 0.614 mole sample of ideal gas at 12 °C occupies a volume of 4.3 L. What is the pressure of the gas?
8 Helium gas has a molar mass of 2 g/mol. Oxygen gas has a molar mass of 32 g/mol.
How much faster or slower would oxygen effuse from a small opening than helium?
9 What is the average velocity of nitrogen gas molecules at STP?
Molar mass of nitrogen = 14 g/mol
10 A 60.0 L tank of chlorine gas at 27 °C and 125 atm springs a leak. When the leak was discovered, the pressure was reduced to 50 atm. How many moles of chlorine gas escaped?
Balancing Equations Test Questions
1 Balance the following equation:
__ SnO2 + __ H2 → __ Sn + __ H2O
2 Balance the following equation:
__ KOH + __ H3PO4 → __ K3PO4 + __ H2O
3 Balance the following equation:
__ KNO3 + __ H2CO3 → __ K2CO3 + __ HNO3
4 Balance the following equation:
__ Na3PO4 + __ HCl → __ NaCl + __ H3PO4
5 Balance the following equation:
__ TiCl4 + __ H2O → __ TiO2 + __ HCl
6 Balance the following equation:
__ C2H6O + __ O2 → __ CO2 + __ H2O
7 Balance the following equation:
__ Fe + __ HC2H3O2 → __ Fe(C2H3O2)3 + __ H2
8 Balance the following equation:
__ NH3 + __ O2 → __ NO + __ H2O
9 Balance the following equation:
__ B2Br6 + __ HNO3 → __ B(NO3)3 + __ HBr
10 Balance the following equation:
__ NH4OH + __ Kal(SO4)2·12H2O → __ Al(OH)3 + __ (NH4)2SO4 + __ KOH + __ H2O
Balancing Chemical Equations Test Questions
1 __ AgI + __ Na2S → __ Ag2S + __ NaI
2 __ Ba3N2 + __ H2O → __ Ba(OH)2 + __ NH3
3 __ CaCl2 + __ Na3PO4 → __ Ca3(PO4)2 + __ NaCl
4 __ FeS + __ O2 → __ Fe2O3 + __ SO2
5 __ PCl5 + __ H2O → __ H3PO4 + __ HCl
6 __ As + __ NaOH → __ Na3AsO3 + __ H2
7 __ Hg(OH)2 + __ H3PO4 → __ Hg3(PO4)2 + __ H2O
|
|
8 __ HClO4 + __ P4O10 → __ H3PO4 + __ Cl2O7
9 __ CO + __ H2 → __ C8H18 + __ H2O
10 __ KClO3 + __ P4 → __ P4O10 + __ KCl
Concentration and Molarity Test Questions
1 What is the molarity of a solution containing 9.478 grams of RuCl3 in enough water to make 1.00 L of solution?
2 What is the molarity of a solution containing 5.035 grams of FeCl3 in enough water to make 500 mL of solution?
What is the molarity of a solution containing 72.9 grams of HCl in enough water to make 500 mL of solution?
What is the molarity of a solution containing 11.522 grams of KOH in enough water to make 350 mL of solution?
5 What is the molarity of a solution containing 72.06 grams of BaCl2 in enough water to make 800 mL of solution?
How many grams of NaCl are required to prepare 100 mL of a solution of 1 M NaCl?
7 How many grams of KMnO4 are required to prepare 1.0 L of a solution of 1.5 M KMNO4?
8 How many grams of HNO3 are required to prepare 500 mL of a 0.601 M HNO3 solution?
What is the volume of a 0.1 M HCl solution containing 1.46 grams of HCl?
10 What is the volume of a 0.2 M AgNO3 solution containing 8.5 grams of AgNO3?
QUESTIONS FOR THE EXAMINE
1. Subject and tasks of chemical thermodynamics. Isolated, closed and open systems.
2. The first law of thermodynamics. Internal energy, heat and work. Isobaric and isochoric thermal processes. Enthalpy.
3. Hess’s law and its corollaries. Standard formation and combustion heat. Thermochemical calculations and their usage for energetic characteristic of biochemical processes.
4. Interconnection between the processes of metabolism and energy exchange. Caloric value of main constituents of food and some food products. Energy consumption at different modes of moving activity.
5. Thermodynamically reversible and irreversible processes. The second law of thermodynamics. Entropy. Statistic and thermodynamic explanation of entropy. Standard entropy.
6. The Gibbs free energy (isobaric-isothermal potential). Enthalpy and entropy factors. Ex - and endergonic processes in the organism.
7. Thermodynamics of chemical equilibrium. Reversible and irreversible reactions. Concept of chemical equilibrium. Constant of the chemical equilibrium. The interconnection between the constant of chemical equilibrium and the Gibbs free energy. Equations of isotherm and isobaric curve of a chemical reaction.
8. Main concepts of chemical kinetics. Simple and complex, homogeneous and heterogeneous reactions. The speed of homogeneous chemical reactions and methods of its measuring.
9. The main postulate of chemical kinetics. The order of reaction and the reaction speed constant. The Law of mass action for the speed of the reaction and its sphere of application.
10. Kinetic equations of the reactions of zero, first and second order. Period of semi-transformation. Molecularity of the reaction.
11. Theory of active collisions. Arrhenius’ equation. Energy of activation. Vant-Hoff’s rule. Temperature coefficient of the reaction speed for enzymatic processes.
12. Osmose and osmotic pressure of solutions. Vant-Hoff’s law.
13. The pressure of saturated vapor of solvent above the solution. Raoul’s first law.
14. Boiling and freezing temperatures of solvents. Raoul’s second law. Cryoscopy. Ebullioscopy.
15. Colligative properties of electrolyte solutions. Isotonic coefficient.
16. The theory of weak electrolyte solutions. The theory of strong electrolyte solutions.
|
|
17. The ion product of water. Hydrogen ion exponent pH.
18. Calculation of solution pH of weak and strong acids and bases.
19. Determination of hydrogen ion exponent.
20. Buffer systems, their classifications. Basic buffer systems of the organism. Acidosis. Alkalosis.
21. Calculation of рН of acid and basic buffer solutions. Mechanism of action of buffer systems.
22. Titration analysis, its methods and tasks. Classification of titration analysis methods.
23. Requirements to the methods used in titration analysis. Standard solutions. Primary standards and requirements made to them. Secondary standards. Calculations in titration analysis.
24. Acid-base titration. Application of the method. The essence and methods of acid-base titration. Equivalence point at acid-base titration. Acid-base indicators. Physical-chemical characteristics of acid-base indicators. Curves of acid-base titration. Choice of the indicator. Standardization of titrants in methods of acid-base titration.
25. Oxidation-reduction titration. Main postulates of the electron theory of oxidation-reduction processes. General characteristic and classification of oxidation-reduction titration methods. Curves of oxidiometric titration.
26. The electron shell of the atom. Energy levels. The wave nature of microparticles motion. The uncertainty principle. The electron cloud.
27. The quantum numbers. The principal quantum number n. The angular momentum quantum number ℓ. Magnetic quantum number mℓ. Electron spin. The shapes of atomic orbitals.
28. Electron configurations of the elements. The Pauly principle. Hund's rule. Order of orbital energies and assignments. Orbital energies. Electron configurations of the main group elements. Electron configurations of the transition elements.
29. The periodic table. Atomic properties and periodic trends. Atomic size. Ionization energy. Electron affinity.
30. Chemical bond formation.Valence electrons. The formation of a covalent bond in H2 molecule.
31. Properties of covalent bond. The bond order. Single and multiple bonds. Sigma and π-bond. The donor–accepter mechanism of formation of covalent bond. Bond length. Bond energy.
32. Polarity and electronegativity. Oxidation numbers. Ionic bond.
33. The structure of complex compounds: inner sphere, outer sphere, central atom, ligands, coodination number, dentation of ligands.
34. Classification and nomenclature of complex compounds. Cyclic complexes or chelates.
35. Dissociation of complex compounds in solutions. Destruction of complex compounds.
36. Equilibria and the processes in solutions with complex compounds.
37. Electrode and oxidation-reduction (OR) potentials, the mechanism of their appearance and the dependence on different factors. Nernst-Peters' equation for calculating of potential values.
38. Galvanic elements (chemical and concentration): action mechanism and calculation of EMF. Measuring of electrode and OR-potentials.
39. Reversible electrodes of the first and the second type (hydrogen and chlorine-silver). Ion-selective electrodes (glass electrode). Structure and mechanism of potential origin.
40. Special features of the energy state of phase interfaces. Surface energy and surface tension.
41. Surface active and surface inactive substances. Surface tension isotherms. Ducklo-Traube rule. Stalagmometric method of measurement of surface tension in liquids.
42. Adsorption at the interface liquid-gas and liquid-liquid. Gibbs’ equation and its analysis. Molecule orientation in the surface layer; the structure of the lipid biolayer of biological membranes.
43. Adsorption on the interface solid-gas and solid-liquid. Langmure and Friendlikh’s adsorption isotherms. Langmure’s and Friendlikh’s equations, their analysis.
44. Dispersion systems, their peculiarities and classification.
45. Molecular-kinetic properties of colloidal systems. Sedimentation. Optical properties of colloidal systems. Opalescence.
46. Structure of colloid particles. Methods of obtaining and purification of colloidal systems. Peptization.
|
|
47. Kinds and factors of colloidal system stability. Coagulation of colloidal solutions and factors causing it.
48. Coagulation of colloidal solutions by electrolytes. Coagulation threshold. Kinetics and coagulation mechanism of colloidal solutions.
49. Coagulation processes at purification of drinking water and sewage.
50. Colloid protection and its importance.
Subject of papers
|
|
|

Организация стока поверхностных вод: Наибольшее количество влаги на земном шаре испаряется с поверхности морей и океанов (88‰)...

Индивидуальные и групповые автопоилки: для животных. Схемы и конструкции...
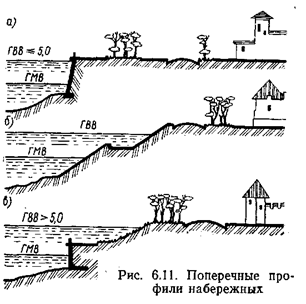
Поперечные профили набережных и береговой полосы: На городских территориях берегоукрепление проектируют с учетом технических и экономических требований, но особое значение придают эстетическим...
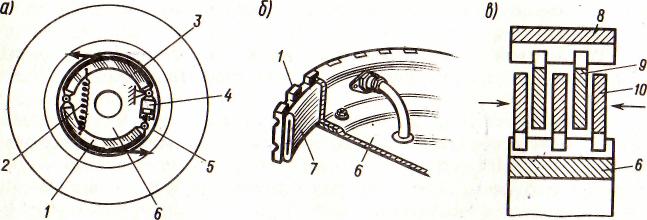
Автоматическое растормаживание колес: Тормозные устройства колес предназначены для уменьшения длины пробега и улучшения маневрирования ВС при...
© cyberpedia.su 2017-2024 - Не является автором материалов. Исключительное право сохранено за автором текста.
Если вы не хотите, чтобы данный материал был у нас на сайте, перейдите по ссылке: Нарушение авторских прав. Мы поможем в написании вашей работы!