The term "redox" comes from two concepts involved with electron transfer: reduction and oxidation.[1] It can be explained in simple terms:
Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion.
Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, these are only specific examples of a more general concept of reactions involving electron transfer.
Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid–base reactions. Like acid–base reactions, redox reactions are a matched set, that is, there cannot be an oxidation reaction without a reduction reaction happening simultaneously. The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge.
Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as "redox" even though no electron transfer occurs (such as those involving covalent bonds
A half-reaction in which a chemical species decreases its oxidation number, usually by gaining electrons.
The H+ ions, with an oxidation number of +1, are reduced to H2, with an oxidation number of 0, in the reaction: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g).
This is an introduction to oxidation-reduction reactions, also known as redox reactions. Learn what redox reactions are, get examples of oxidation-reduction reactions, and find out why redox reactions are important.
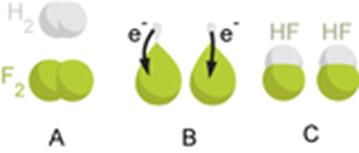
A good example is the reaction between hydrogen and fluorine in which hydrogen is being oxidized and fluorine is being reduced:
H2 + F2 → 2 HF
We can write this overall reaction as two half-reactions:
the oxidation reaction:
H2 → 2 H+ + 2 e−
and the reduction reaction:
F2 + 2 e− → 2 F−
Analyzing each half-reaction in isolation can often make the overall chemical process clearer. Because there is no net change in charge during a redox reaction, the number of electrons in excess in the oxidation reaction must equal the number consumed by the reduction reaction (as shown above).
Elements, even in molecular form, always have an oxidation state of zero. In the first half-reaction, hydrogen is oxidized from an oxidation state of zero to an oxidation state of +1. In the second half-reaction, fluorine is reduced from an oxidation state of zero to an oxidation state of −1.
When adding the reactions together the electrons are canceled:
H2 → 2 H+ + 2 e−
F2 + 2 e− → 2 F−
H2 + F2 → 2 H+ + 2 F−
And the ions combine to form hydrogen fluoride:
2 H+ + 2 F− → 2 HF
The overall reaction is:
H2 + F2 → 2 HF
The reaction between hydrogen gas and fluorine gas to form hydrofluoric acid is an example of a redox reaction or oxidation-reduction reaction.
In oxidation-reduction or redox reactions, it is important to be able to identify which atoms are being oxidized and which atoms are being reduced. To identify if an atom is either oxidized or reduced, you only have to follow the electrons in the reaction.
Example Problem:
Identify the atoms that were oxidized and which atoms were reduced in the following reaction:
Fe2O3 + 2 Al → Al2O3 + 2 Fe
The first step is to assign oxidation numbers to each atom in the reaction. The oxidation number of an atom is the number of unpaired electrons available for reactions.
Review: Rules for Assigning Oxidation Numbers
Fe2O3:
The oxidation number of an oxygen atom is -2. 3 oxygen atoms has a total charge of -6. To balance this, the total charge of the iron atoms must be +6. Since there are two iron atoms, each iron must be in the +3 oxidation state. To summarize: -2 electrons per oxygen atom, +3 electrons for each iron atom.
2 Al: The oxidation number of a free element is always zero.
Al2O3:
Using the same rules for Fe2O3, we can see there are -2 electrons for each
oxygen atom and +3 electrons for each aluminum atom.
2 Fe: Again, the oxidation number of a free element is always zero.
Put all this together in the reaction, and we can see where the electrons went:
Iron went from Fe3+ on the left side of the reaction to Fe0 on the right. Each iron atom gained 3 electrons in the reaction.
Aluminum went from Al0 on the left to Al3+ on the right. Each aluminum atom lost three electrons.
Oxygen stayed the same on both sides. With this information, we can tell which atom was oxidized and which atom was reduced. There are two mnemonics to remember which reaction is oxidation and which reaction is reductions.
Back to our case: Iron gained electrons so iron was oxidized. Aluminum lost electrons so aluminum was reduced.
Oxidation and reduction are two types of chemical reactions that often work together. Oxidation and reduction reactions involve an exchange of electrons between reactants. For many students, the confusion occurs when attempting to identify which reactant was oxidized and which reactant was reduced. What is the difference between oxidation and reduction?
Oxidation occurs when a reactant loses electrons during the reaction. Reduction occurs when a reactant gains electrons during the reaction. This often occurs when metals are reacted with acid. Consider the reaction between zinc metal and hydrochloric acid.
Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g)
If this reaction where broken down to the ion level:
Zn(s) + 2 H+(aq) + 2 Cl-(aq) → Zn2+(aq) + 2 Cl-(aq) + 2 H2(g)
First, look at what happens to the zinc atoms. Initially, we have a neutral zinc atom. As the reaction progresses, the zinc atom loses two electrons to become a Zn2+ ion.
Zn(s) → Zn2+(aq) + 2 e-
The zinc was oxidized into Zn2+ ions. This reaction is an oxidation reaction.
The second part of this reaction involves the hydrogen ions. The hydrogen ions are gaining electrons and bonding together to form dihydrogen gas.
2 H+ + 2 e- → H2(g)
The hydrogen ions each gained an electron to form the neutrally charged hydrogen gas. The hydrogen ions are said to be reduced and the reaction is a reduction reaction. Since both processes are going on at the same time, the initial reaction is called an oxidation-reduction reaction. This type of reaction is also called a redox reaction (REDuction/OXidation).
You could just memorize oxidation: lose electrons-reduction: gain electrons, but there are other ways. There are two mnemonics to remember which reaction is oxidation and which reaction is reductions. The first one
is OIL RIG:
O xidation I nvolves L oss of electrons
R eduction I nvolves G ain of electrons.
The second is "LEO the lion says GER".
L ose E lectrons in O xidation
G ain E lectrons in R eduction.
Oxidation and reduction reactions are common when working with acids and bases and other electrochemical processes. Use these two mnemonics to help keep in mind which process is the oxidation and which is the reduction reaction.