These two features (to play) an important role in the mechanical behavior of materials.
For instance, small amounts of sulphide particles, such as grey manganese sulphide (MnS), (to be) usually tolerable in steels.
Sulphide films at the grain boundaries (to cause) unacceptable embrittlement.
Alloys (to be) rarely cooled or heated at very slow rates.
Quenching, as practised in the heat-treatment of steels, can (to produce) metastable phases known as martensite and bainite.
6. Join the halves of the sentences in the columns:
| They frequently exist over a range of composition and
| a convenient means to divide a complex phase diagram (binary or ternary) into more readily understandable parts.
|
| A congruently melting phase provides
| having a complex crystal structure.
|
| In general, intermediate phases are hard and brittle,
| be dispersed evenly as particles throughout the α-grains.
|
| The β-phase may
| it is therefore generally advisable to avoid the term ‘compound’.
|
7. Answer the questions:
Does an intermediate phase differ in crystal structure from the primary phases?
Sometimes intermediate phases have definite stoichiometric ratios of constituent atoms, don’t they?
3. Why is it generally advisable to avoid the term ‘compound’ towards intermediate phases?
Are phase diagrams extremely useful in the interpretation of metallic and ceramic structures?
What is the most serious limitation of phase diagrams?
Phase diagrams portray only equilibrium states, don’t they?
8. Read text B and choose the best title for it:
The difficulty of achieving furnace temperatures.
The versatile behaviour of steels during heat-treatment.
3. Iron–carbon system.
Make sure that you know the following words and word combinations:
ferrous alloys - железные сплавы
cast iron – чугун
furnace – печь, топка
austenite and ferrite - аустенит и феррит
cementite - карбид железа
Text B
The diagram for the part of the Fe–C system shown in Figure 3.21 is the basis for understanding the microstructures of the ferrous alloys known as steels and cast irons. Dissolved carbon clearly has a pronounced effect upon the liquidus, explaining why the difficulty of achieving furnace temperatures of 1600°C caused large-scale production of cast irons to predate that of steel. The three allotropes of pure iron are α-Fe (bcc), γ-Fe (fcc) and δ-Fe (bcc). Small atoms of carbon dissolve interstitially in these allotropes to form three primary solid solutions: respectively, they are α-phase (ferrite), γ-phase (austenite) and δ-phase. At the other end of the diagram is the orthorhombic intermediate phase  C, which is known as cementite.
C, which is known as cementite.
The large difference in solid solubility of carbon in austenite and ferrite, together with the existence of a eutectoid reaction, are responsible for the versatile behaviour of steels during heat-treatment. 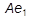 ,
,  ,
, 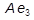 and
and 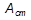 indicate the temperatures at which phase changes occur: they are arrest points for equilibria detected during thermal analysis. For instance, slow cooling enables austenite (0.8% C) to decompose eutectoidally at the temperature
indicate the temperatures at which phase changes occur: they are arrest points for equilibria detected during thermal analysis. For instance, slow cooling enables austenite (0.8% C) to decompose eutectoidally at the temperature 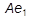 and form the microconstituent pearlite, a lamellar composite of soft, ductile ferrite (initially 0.025% C) and hard, brittle cementite (6.67% C). Quenching of austenite from a temperature above
and form the microconstituent pearlite, a lamellar composite of soft, ductile ferrite (initially 0.025% C) and hard, brittle cementite (6.67% C). Quenching of austenite from a temperature above  forms a hard metastable phase known as martensite. From the diagram one can see why a medium-carbon (0.4%) steel must be quenched from a higher
forms a hard metastable phase known as martensite. From the diagram one can see why a medium-carbon (0.4%) steel must be quenched from a higher  temperature than a high-carbon (0.8%) steel. Temperature and composition ‘windows’ for some important heat-treatment operations have been superimposed upon the phase diagram.
temperature than a high-carbon (0.8%) steel. Temperature and composition ‘windows’ for some important heat-treatment operations have been superimposed upon the phase diagram.
Part VI
1. Learn the words:
| vacancy
| пустота, полость
|
| adjacent
| примыкающий, смежный
|
| to arise
| возникать
|
| to adjoin
| граничить
|
| probability
| вероятность
|
| Maxwell–Boltzmann distribution law
| закон распределения Максвелла Больцмана
|
| velocity
| скорость
|
| slope
| наклон, уклон
|
| Arrhenius equation
| уравнение Аррениуса
|
| entropy of activation
| энтропия активации
|
| transition
| переход
|
| magnitude
| величина
|
| intercept
| пересечение
|
| temperature-independent terms
| температурно-независимые термины
|
| the frequency factor
| частотный коэффициент
|
| a process of nucleation and growth
| процесс зарождения и роста
|
| chance thermal fluctuations
| возможные тепловые колебания
|
| nucleus (nuclei)
| ядро (ядра)
|
| at the expense of
| за счет
|
| the rate of nucleation
| скорость зарождения
|
| the rate of growth
| темпы роста
|
| sluggishness
| медлительность
|
| hysteresis
| гистерезис (отставание, запаздывание)
|
| to induce
| вызывать
|
| to oppose
| противостоять
|
| strain energy
| энергия деформации
|
| surface energy
| поверхностная энергия
|
| spherical nucleus
| сферическое ядро
|
| misfit strain energy
| энергия несоответствия
|
| driving force
| движущая сила
|
| mismatch
| несоответствие
|
| misfit dislocations
| дислокации несоответствия
|
| to relieve
| освобождать
|
| grid
| сетка
|
| Burgers vector
| Бюргерский вектор
|
| to overlap
| частично совпадать
|
| discrete nature
| дискретный характер
|
2. Guess the meaning of the following words and word combinations:
Kinetic considerations; atomic migration; diffusion; the potential barrier; the temperature-dependence of the reaction rate; entropy term; the intermediate configuration of higher free energy; atomically thin layers; nucleation and growth transformation; rate-controlling.
3. Identify the part of speech and translate the words:
To occur, occurrence; to favour, favourable; to vary, varied, various; to respect, respectful, respective, respectively; probable, probably, probability; to measure, measured, measurement; to transit, transition, transitional; to express, expressed, expression; to localize, localized, localizer; sluggishness, sluggishly, sluggish; to induce, induced, inducement; to oppose, opposed, opposition.
4. Match the words with their definitions:
| redistribution of the atoms
| межатомные силы между движущимся атомом и группой атомов
|
| empty adjacent atomic site
| энтальпии активации для нагрева и охлаждения
|
| lattice site
| с достаточной энергией, чтобы перепрыгнуть через барьер
|
| interatomic forces between the moving atom and the group of atoms
| энергия на атом в электронных вольтах
|
| the activation enthalpies for heating and cooling
| скорость реакции определяется скоростью
|
| having sufficient energy to jump the barrier
| частота вибрации
|
| the energy per atom in electron volts
| узел решетки
|
| the rate of reaction is given by Rate
| пустой соседний атомный узел
|
| the frequency of vibration
| перераспределение атомов
|
Text A


 C, which is known as cementite.
C, which is known as cementite.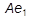 ,
,  ,
, 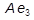 and
and 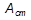 indicate the temperatures at which phase changes occur: they are arrest points for equilibria detected during thermal analysis. For instance, slow cooling enables austenite (0.8% C) to decompose eutectoidally at the temperature
indicate the temperatures at which phase changes occur: they are arrest points for equilibria detected during thermal analysis. For instance, slow cooling enables austenite (0.8% C) to decompose eutectoidally at the temperature  forms a hard metastable phase known as martensite. From the diagram one can see why a medium-carbon (0.4%) steel must be quenched from a higher
forms a hard metastable phase known as martensite. From the diagram one can see why a medium-carbon (0.4%) steel must be quenched from a higher