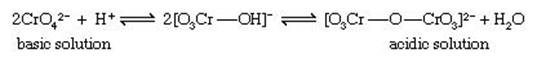The amphoteric metals of groups VB (vanadium, niobium, and tantalum) and VIB (chromium, molybdenum, and tungsten) in the +5 and +6 oxidation states, respectively, form weak acids that readily condense (polymerize) to form anions containing several molecules of the acidanhydride. If these condensed acids contain only one type of acid anhydride, they are called isopoly acids, and their salts are called isopoly salts. The acid anhydrides also can condense with other acids (e.g., phosphoric or silicic acids) to form heteropoly acids, which can form heteropoly salts. The condensation reactions, which occur reversibly in dilute aqueous solution, involve formation of oxo bridges by elimination of water from two molecules of the weak acid. The best-known and simplest example is the condensation of yellow chromate ion (CrO42−) to form the orange isopoly dichromate ion (Cr2O72−), an equilibriumreaction the extent of which depends on the pH. In acidic solution the isopoly anion Cr2O72−, predominates while in basic solution the simple ion CrO42− predominates.
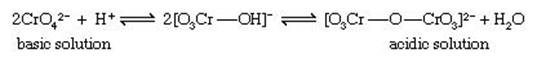
Heteropoly acids and their salts may be formed by coordination of the central atom with four to six oxo anions, which may be mononuclear (containing one metal ion each), as in H7[P(MoO4)6], or trinuclear (containing three metal ions each), as in H3[P(W3O10)4]. Incomplete replacement of oxygen atoms in PO43− ions by MoO3 groups can result in dimers (two-molecule polymers), as, for example, {OP[O(MoO3)3]3}26−. About 70 elements can act as central (hetero) atoms in heteropoly anions. Because each element may form more than one heteropoly anion and some of these anions can contain several different heteroatoms, thousands of heteropoly acids exist. Heteroatoms may be primary (these atoms are essential to the polyanion structure and thus not susceptible to chemical exchange) or secondary (these atoms can be removed by chemical reaction from the polyanion structure without destroying it). Heteropoly anions can be regarded as coordination compounds with polyanion ligands; e.g., [(H3N)5Cr(OH2)]3+ can be considered the parent of [(SiW11O39)Cr(OH2)]5−.
A variety of synthetic procedures are available for the preparation of isopoly acids and salts, which are usually less stable than heteropoly compounds. Heteropolymolybdates and heteropolytungstates are always prepared in solution, usually after acidifying and heating the theoretical amounts of reactants. In general, free heteropoly acids and salts, of which the heteropolymolybdates and heteropolytungstates are the best known, have very high molecular weights (some above 4,000) as compared with other inorganic electrolytes, are very soluble in water and organic solvents, are almost always highly hydrated with several hydrates existing, and are highly coloured. Some are strong oxidizing agents that can be reduced to stable, intensely deep blue species (heteropoly blues), which in turn can act as reducing agents, restoring the original colour on oxidation. The stoichiometry, oxidation-reduction potentials, and other characteristics of these reactions have been investigated by various methods. The free acids, which are polyprotic (contain several replaceable hydrogen ions), are fairly strong and nearly always stronger than the corresponding acids from which they are derived.
All heteropolymolybdate and heteropolytungstate anions are decomposed in strongly basic solution to form simple molybdate or tungstate ions and either an oxy anion or a hydrous metal oxide of the central metal atom, e.g.:
[P2Mo18O62]6− + 34OH− -----> 18MoO42− + 2HPO42− + 16H2O [NiW6O24H6]4− + 8OH– -----> 6WO42− + Ni(OH) 2 + 6H2O
Throughout specific ranges of pH and other conditions, most solutions of heteropolymolybdates and heteropolytungstates appear to contain predominantly one distinct species of anion, many of which are remarkably stable and nonlabile.
The first heteropoly compound, (NH4)3[PMo12O40], was obtained by the Swedish chemist Jöns Jacob Berzelius in 1826 as a yellow, crystalline precipitate, the formation of which is still used for the classical qualitative detection and quantitative estimation of phosphorus (after conversion to phosphate). By the beginning of the 20th century, hundreds of isopoly and heteropoly compounds were reported, many of which were based on incorrect analyses or failure to detect mixed crystals. Formulas were reported in terms of the old Berzelius dualistic theory as a combination of oxides, such as 3Na2O∙Cr2O3∙12MoO3∙20H2O for Na3CrMo6O24H6∙7H2O, and often merely expressed analytical results rather than structure. In addition to their use in analytical chemistry, heteropoly compounds have found use as catalysts, molecular sieves, corrosion inhibitors, photographic fixing agents, and precipitants for basic dyes.
Few structural studies of such compounds were carried out, but this lack did not prevent the elaboration of various unsuccessful theories to account for their structures. In 1907 Werner applied his coordination theory to the structure of 12-tungstosilicic acid, H4 [SiW12O40], and its salts by assuming that the central group is an SiO44− ion surrounded octahedrally by six RW2O6+ groups (R = a unipositive ion), four linked by primary (ionic) and two linked by secondary (coordinate covalent) valences. Difficulties were encountered by this system as well as by the later (1910–21), more elaborate Miolati-Rosenheim theory. Modern conclusive knowledge of the structures of heteropoly compounds did not begin until 1934, with J.F. Keggin’s determination of the structure of H3 [PO4W12O36]∙5H2O by the most direct means, X-ray diffraction.
Advertisement
The structures of isopoly and heteropoly compounds consist of polyhedrons sharing corners and edges with one another. In heteropolymolybdates or heteropolytungstates, each molybdenum or tungsten atom is located at the centre of an octahedron, each vertex of which is occupied by an oxygen atom. These octahedrons can share corners or edges or both with other MoO6 or WO6 octahedrons. In [Mo8O26]4− eight MoO6 octahedrons share edges. In [PMo12O40]3− the central phosphorus atom is located at the centre of a PO4 tetrahedron, which is surrounded by 12 MoO6 octahedrons, which share corners so that the correct number of oxygen atoms is utilized.
30 Describe substitution reactions in inner sphere Na[Al(OH)4] +SiO4 4- =Na2O• Al2O3•SiO2 •H2O and indicate possible forming compounds.