| Zn – zinc
| O2 – oxygen
|
| HCl – hydrochloric acid
| BaCl2 – barium chloride
|
| KMnO4 - potassium permanganate
| CaO – calcium oxide
|
| K2MnO4 - potassium manganate
| H2SO4 – sulphuric acid
|
| ZnCl2 – zinc chloride
| CO2 – carbon dioxide
|
| MnO2 – manganese oxide (IV)
| BaSO4 – barium sulphate
|
| H2 – hydrogen
| CaCO3 – calcium carbonate
|
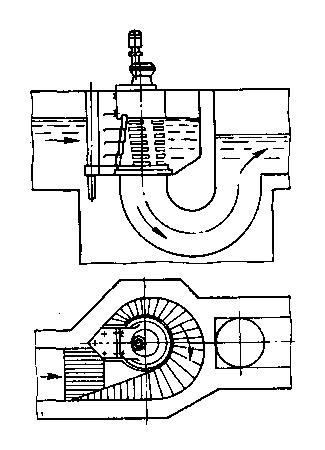
Look at the picture and comment on it from the point of view of chemistry.

Replace the underlined words with their English equivalents from the list. Give the general idea of the text in 2-3 sentences.
| fertilizers
|
| petroleum refining
|
| pickling (cleaning)iron
rayon
battery acid
impurities
|
USES OF SULPHURIC ACID
 Sulphuric acid is one of the most important industrial chemicals. More of it is made each year than is made of any other manufactured chemical. It has widely varied uses and plays some part in the production of nearly all manufactured goods. The major use of sulphuric acid is in the production of удобрений, e.g., superphosphate of lime and ammonium sulfate. It is widely used in the manufacture of chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, and drugs. It is used in очистке нефти to wash примеси out of gasoline and other refinery products.
Sulphuric acid is one of the most important industrial chemicals. More of it is made each year than is made of any other manufactured chemical. It has widely varied uses and plays some part in the production of nearly all manufactured goods. The major use of sulphuric acid is in the production of удобрений, e.g., superphosphate of lime and ammonium sulfate. It is widely used in the manufacture of chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, and drugs. It is used in очистке нефти to wash примеси out of gasoline and other refinery products.
Sulphuric acid is used in processing metals, e.g., in травлении iron and steel before plating them with tin or zinc. Вискоза is made with sulfuric acid. It serves as the electrolyte in the lead-acid storage battery commonly used in motor vehicles (acid for this use, containing about 33% H2SO4 and with specific gravity about 1.25, is often called аккумуляторной кислотой).
Watch the video 'Sulphuric Acid Production'. Decide if the sentences are true (T) or false (F).

| 1. Sulphuric acid is used in the drug production.
| |
| 2. The small plant makes 60 tons of acid per day.
| |
| 3. Sulphur is transported as a liquid around 200 degrees Celsius.
| |
| 4. In the converter sulphur dioxide reacts with oxygen.
| |
| 5. The catalyst is the most effective at 840 degrees Celsius.
| |
| 6. About 95% of the original SO2 has been converted to SO3.
| |
| 7. The acid concentration must be about 98% at the final stage.
| |
Explain the following words and word-combinations in English.
| Reactant
| Electrolysis
|
| Product
| Neutralization reactions
|
| Breakdown
| Redox reactions
|
Make up the sentences of your own with the words in bold of the text B. Provide your own examples of combustion reactions and precipitation reactions.
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Project Work
 8. Prepare a report upon one of the following topics:
8. Prepare a report upon one of the following topics:
1. Inorganic chemistry as a science.
2. Differences and similarities between inorganic and organic chemistry.
3. Types of inorganic chemical reactions.
4. The Periodic table as the basis of inorganic chemistry.
9. Study the web site www.sciencedaily.com and prepare a report or presentation on inorganic chemistry news. Share the information with the groupmates.

|
UNIT 3. ANALYTICAL CHEMISTRY
|
I. Lead-in
1.  What topics does analytical chemistry deal with?
What topics does analytical chemistry deal with?
2. What is the modern definition of analytical chemistry?
3. What are the two main types of chemical analysis?
4. What methods (techniques) of analysis do you know?


 Sulphuric acid is one of the most important industrial chemicals. More of it is made each year than is made of any other manufactured chemical. It has widely varied uses and plays some part in the production of nearly all manufactured goods. The major use of sulphuric acid is in the production of удобрений, e.g., superphosphate of lime and ammonium sulfate. It is widely used in the manufacture of chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, and drugs. It is used in очистке нефти to wash примеси out of gasoline and other refinery products.
Sulphuric acid is one of the most important industrial chemicals. More of it is made each year than is made of any other manufactured chemical. It has widely varied uses and plays some part in the production of nearly all manufactured goods. The major use of sulphuric acid is in the production of удобрений, e.g., superphosphate of lime and ammonium sulfate. It is widely used in the manufacture of chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, and drugs. It is used in очистке нефти to wash примеси out of gasoline and other refinery products. 8. Prepare a report upon one of the following topics:
8. Prepare a report upon one of the following topics:
 What topics does analytical chemistry deal with?
What topics does analytical chemistry deal with?