To solve equations (35) and (40) need to know the values of the mean molar heat capacities of gases m c. Unlike heats, referred to 1 kg. average molar heat capacity for all diatomic gases have the same value. For the temperature range of 800 - 30000 molar heat capacity medium abc diatomic gases and air at constant volume.
(m c v l ) T = 4,815+0,415*10-3Т kcal / (mol deg)
For carbon dioxide СО2;
(m c v l ) T = 7,465+1,54 *10-3Т kcal / (mol deg)
For water vapor in the range 300 – 15000 абс,
(m c v l ) T = 5,595+0,83 *10-3Т kcal / (mol deg)
In the interval 1500 – 30000;
(m c v l ) T = 3,615+2,16 *10-3Т kcal / (mol deg)
The average molar heat capacity of gas at constant pressure:
(m c p )=(m c v)+1,985 kcal / (mol deg)
where 1,985 kilocalories - work at a constant pressure of one mole of gas under heating at 10, expressed in calories.
The enlargement process.
Gaza, extending inside the cylinder, perform useful work. The enlargement process is displayed on the indicator diagram (Fig. 9) line z1zb, extension of the line z1z is called the pre-expansion, the expansion of the line z-b, followed. During the expansion process is a variable heat transfer. At the beginning of the expansion process occurs fuel has burnt out, whereby gases get warm. For all the expansion process gases give off heat to the cylinder walls, the surface area of which at least the stroke increases. gas temperature at the expansion process decreases; therefore, measured the temperature difference between the gases and cylinder walls. From the temperature difference of heat transfer relationship. Moreover, gas leakage occurs through gaps of piston rings. The process of further enlargement is a polytropic process, the polytropic index in the process of expanding all the time changes. Changes in the polytropic expansion depending on the stroke, draw through two curves current z: adiabatic and the isotherm (Fig. 10).
Since the adiabatic expansion of the gases are cooled, in order to carry out the process of expansion isotherm, gases need to give warmth. Curve expansion isotherm zb1 a hollow. Consequently, the application of heat to the gas expansion curve will be flatter, and the polytropic exponent, tending to unity, will decrease. During rebound, the heat curve will pass more steeply, and the polytropic index will increase..
When expanding gases are cooled, their heat capacity decreases; therefore, when the process of expanding the adiabatic index increases
k 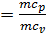 =1+1,985/
=1+1,985/  , and together with him will increase and the corresponding polytropic index. If you change the speed limit will change the flow time of gas leakage process. With increasing speed the polytropic index will decrease. At the start of the expansion polytropic index as a consequence of fuel has burnt out will have a smaller value, as the motion of the piston to BDC polytropic index will increase. 11 given the nature of the change indicators polytropic expansion in the course of the piston.
, and together with him will increase and the corresponding polytropic index. If you change the speed limit will change the flow time of gas leakage process. With increasing speed the polytropic index will decrease. At the start of the expansion polytropic index as a consequence of fuel has burnt out will have a smaller value, as the motion of the piston to BDC polytropic index will increase. 11 given the nature of the change indicators polytropic expansion in the course of the piston.
use the value of the average polytropic expansion When carrying out thermal calculations. The median polytropic expansion is called a constant-highest level at which gases expands, making the same work as in the variables. In the presence of the indicator diagram, taken from a running engine, the average value of the polytropic index extension is determined analytically or graphically on the 6 (cm) Average polytropic n2 polytropic expansion following expression:
n2 = 1.22 + 130/ n, (41)
n - where the engine speed.
This relationship has been established on the basis of studies of indicator chart carburetor engines. For diesel engines given the relatively stronger after-burning can be encouraged to reduce the n2 value obtained by the expression (41), at 0.01-0.02.
The pressure in the expansion end (point b indicator diagram, Figure 9) defined by the polytropic equation;
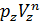 =
=  ,
,
 =
= 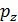 (
( )n.
)n.
For petrol engines  =
=  , consequently;
, consequently;
 =
= 
 (42)
(42)
For diesel engines  =
=  , consequently;
, consequently;
 =
= 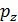 (
( )n
)n  (43)
(43)
Temperature Tb it is easily determined from the characteristic equations:
 =GRTb,
=GRTb,
 =GRTz ,
=GRTz ,
Dividing one equation to the other, we have:
 =
= 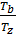 ; as
; as  n ,
n ,
at 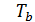 =
= 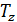 (
( )n-1 =
)n-1 = 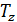 (
( n – 1 (44)
n – 1 (44)
For petrol and gasoline engine r =1, consequently, for this is engines
 =
=  (45)
(45)
Exhaust process.
As was pointed out when considering the absorption process, the exhaust valve is opened well ahead, closed with a certain delay, ie after passing through the piston TDC. At the beginning of the opening of the exhaust valve the pressure inside the cylinder typically has a value of 3-5 atm. The ratio of the pressure value inside the cylinder to the pressure medium is always greater than the outer two. This ratio is critical, therefore, at the beginning of expiration gas inlet critical process proceeds with a velocity equal to the velocity of sound in the medium, and is independent of the pressure difference. During this period, the exhaust gases reach the speed of 400 m / sec. When the pressure inside the cylinder to fall to 2 atm, which occurs in the vicinity of BDC, the exhaust velocity drops sharply and will depend on the pressure difference; During this period the speed of the exhaust valve under the head is 60-100 m / sec.
Just as the intake system, exhaust system must be designed to aerodynamic resistance was small as possible channel surface should be smooth, smooth turns Setting muffler significantly increases resistance and causes a decrease in power. Pressure Pr at the end of release depends on the engine speed. We can assume that Pr = p0 (1 +0,55*10-4n) кг/см2
Temperature Тr end of release (point r indicator diagram) for gasoline engines can be assumed to be equal
Тr = 800-10000 абс.
For diesel engine:
Тr = 700-9000 абс.
The mean indicated pressure
Mean indicated pressure pi It called a conditional permanent excess pressure at which gas per stroke might make the same work that they do at alternating pressure in one cycle To derive the expression the mean indicated pressure pi , to start, consider a simplified diagram displays only the process: the compression and expansion (fig12). The area acz1 zba indicator diagram gives gas work per cycle If this area turn into isometric rectangle with the same base Vh , the height of the rectangle and gives the mean indicated pressure for the process taken by us The value of the area of the indicator diagram obtained if the square kz1zbdk, relevant work of expansion of gases, subtract the area ackda, corresponding to the work done in compression. The area kz1zbdk, It consists of two parts: the area kz1zlk, related work L z1-z pre-expansion, and area lzbdl, related work L x-b pre-expansion:
L z1-z = pz Vz – pz Vc ,
or
L z1-z = pc Vc l (r-1), (46)
as Pz = l pc Vz = r Vc
For the preparation of the work of the equation L x-b polytropic expansion of gases derive the equation works for any polytropic process Let the curve 1-2 (Fig 13) displays a polytropic process p v n=const. The works L 1- 2 It will be expressed by an area 1-2-3-4
L 1- 2 =  ;
;
As p1 V12=p2V2n= const =c, the p =  where p in kg /m3, V in m 3,
where p in kg /m3, V in m 3,
Consequently:
L 1- 2 =с  =
=  =
=  (V2 1-n – V1 1-n)=
(V2 1-n – V1 1-n)= 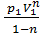 (V1 1-n – V2 1-n)=
(V1 1-n – V2 1-n)=
=  [ 1 – (V1/ V2 )n-1]. (47)
[ 1 – (V1/ V2 )n-1]. (47)
Based on the expression (47) we write the equation of work L z – b, pre-expression:
L z – b = 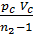 [1- (Vz/Vb)n-1] =
[1- (Vz/Vb)n-1] =  lr[1- (
lr[1- ( )n-1] (48)
)n-1] (48)
Similarly, work
L а –с = 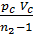 [1- (Vа/Vс)n-1] =
[1- (Vа/Vс)n-1] =  lr[1- (
lr[1- ( )n-1] (49)
)n-1] (49)
On the basis of expressions (46), (47) and (49) the work under review cycle will be equal Lcycle = pc Vc { l (r - 1)+  [1-(
[1-( 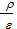 )n-1 ] -
)n-1 ] - 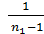 (1-
(1- 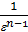 )}
)}  . (50)
. (50)
Dividing the work L cycle by the amount Vh and consider that  =
= 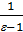 , we obtain the mean indicated pressure: P11=
, we obtain the mean indicated pressure: P11=  { l (r-1)+
{ l (r-1)+  [1-(
[1-( 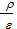 )n-1]-
)n-1]-  (1-
(1- 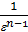 )}
)}
or, for both parts in 10000, we obtain:
P11=  {l (r -1)+
{l (r -1)+  [1-(
[1-( 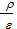 )n-1]-
)n-1]-  (1-
(1- 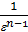 )}
)}  (51)
(51)
For petrol and gasoline engines p=1, consequently:
P11=  {
{  (1-
(1- 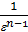 ) -
) -  (1-
(1- 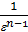 )
)  (52)
(52)
The indicator diagram, taken from a real engine, has no sharp angles, because the combustion process is not actually flows through the line v-const, and p-const, besides opening the valve does not happen instantly FIG 14 shows the indicator diagram of real-carburetor engine, and a dotted line - a simplified diagram. The actual indicator diagram area slightly smaller, so the actual mean indicated pressure will be slightly lower This reduction takes into account a coefficient j = 0,92 – 0,95.
In addition, part of the work is spent on the suction and discharge process. If the area of the work divided into Vh, we get the value of the pressure D р=рк –ра. Therefore, the actual mean indicated pressure is equal to:
р1 = j р11 - D р  (53)
(53)
Mean indicated pressure values in diesel engines р1= 6-10 kg/sm2 for the petrol engines р1= 7 -11kg/sm2. A few less than the mean indicated pressure in diesel engines is obtained by air-fuel ratio
Engine power
Power Indicator
The indicator is called the work capacity of gas cylinders in the engine, performed gases per second.
Working gas per cycle on all cylinders of the engine is equal to:
Lcycle = pi  i kgm/cycle (54)
i kgm/cycle (54)
D – diameter cylinder in cm;
S – the piston stroke length in cm;
i – the number of cylinders
Meaning  S it has a working volume of the cylinder in cm3. The number of cubic centimeters of cylinder volume of 1,000 times the number of liters it: therefore, replacing the expression volume in cm3 of its value in liters, you have to multiply the right-hand side of equation (54) for 1000.
S it has a working volume of the cylinder in cm3. The number of cubic centimeters of cylinder volume of 1,000 times the number of liters it: therefore, replacing the expression volume in cm3 of its value in liters, you have to multiply the right-hand side of equation (54) for 1000.
Lcycle = pi Vл  kgm/cycle
kgm/cycle
where Vл – cylinder engine capacity in liters.
The number of cycles per minute, and τ - the number of cycles In four-stroke engines per minute is equal to the number  , where n – the number of engine revolutions per minute is equal
, where n – the number of engine revolutions per minute is equal  , a two stroke engine equal n. Therefore, the work of the gas is equal to one minute:
, a two stroke engine equal n. Therefore, the work of the gas is equal to one minute:
Lminute = pi Vл 10i  kgm/cycle
kgm/cycle
In one second, that is, the power will be equal to:
Lsek. = pi Vл 10i  kgm/cycle (55)
kgm/cycle (55)
In order to have the value of the indicator of power expressed in horsepower, it is necessary to divide the right side of 75
Ni = pi Vл 10i 
 ;
;
Ni = pi  (56)
(56)


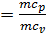 =1+1,985/
=1+1,985/  , and together with him will increase and the corresponding polytropic index. If you change the speed limit will change the flow time of gas leakage process. With increasing speed the polytropic index will decrease. At the start of the expansion polytropic index as a consequence of fuel has burnt out will have a smaller value, as the motion of the piston to BDC polytropic index will increase. 11 given the nature of the change indicators polytropic expansion in the course of the piston.
, and together with him will increase and the corresponding polytropic index. If you change the speed limit will change the flow time of gas leakage process. With increasing speed the polytropic index will decrease. At the start of the expansion polytropic index as a consequence of fuel has burnt out will have a smaller value, as the motion of the piston to BDC polytropic index will increase. 11 given the nature of the change indicators polytropic expansion in the course of the piston.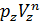 =
=  ,
, =
= 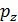 (
( )n.
)n. =
=  , consequently;
, consequently; =
= 
 (42)
(42) =
=  , consequently;
, consequently; )n
)n  =GRTb,
=GRTb, =GRTz ,
=GRTz , =
= 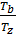 ; as
; as  n ,
n ,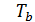 =
= 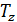 (
( n – 1 (44)
n – 1 (44) =
=  (45)
(45) ;
; where p in kg /m3, V in m 3,
where p in kg /m3, V in m 3, =
=  =
=  (V2 1-n – V1 1-n)=
(V2 1-n – V1 1-n)= 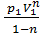 (V1 1-n – V2 1-n)=
(V1 1-n – V2 1-n)= [ 1 – (V1/ V2 )n-1]. (47)
[ 1 – (V1/ V2 )n-1]. (47)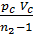 [1- (Vz/Vb)n-1] =
[1- (Vz/Vb)n-1] =  lr[1- (
lr[1- ( )n-1] (48)
)n-1] (48) [1-(
[1-( 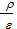 )n-1 ] -
)n-1 ] - 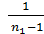 (1-
(1- 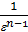 )}
)}  . (50)
. (50) =
= 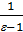 , we obtain the mean indicated pressure: P11=
, we obtain the mean indicated pressure: P11=  { l (r-1)+
{ l (r-1)+  [1-(
[1-(  (1-
(1-  (1-
(1- 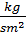 (52)
(52) i kgm/cycle (54)
i kgm/cycle (54) S it has a working volume of the cylinder in cm3. The number of cubic centimeters of cylinder volume of 1,000 times the number of liters it: therefore, replacing the expression volume in cm3 of its value in liters, you have to multiply the right-hand side of equation (54) for 1000.
S it has a working volume of the cylinder in cm3. The number of cubic centimeters of cylinder volume of 1,000 times the number of liters it: therefore, replacing the expression volume in cm3 of its value in liters, you have to multiply the right-hand side of equation (54) for 1000. kgm/cycle
kgm/cycle , where n – the number of engine revolutions per minute is equal
, where n – the number of engine revolutions per minute is equal  , a two stroke engine equal n. Therefore, the work of the gas is equal to one minute:
, a two stroke engine equal n. Therefore, the work of the gas is equal to one minute: kgm/cycle
kgm/cycle kgm/cycle (55)
kgm/cycle (55) ;
; (56)
(56)