
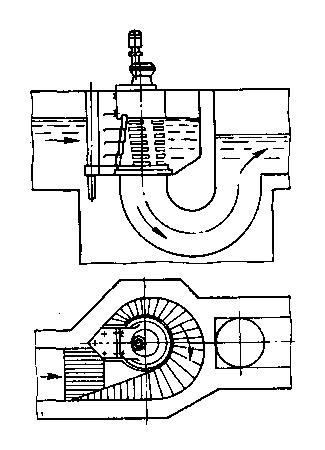
Состав сооружений: решетки и песколовки: Решетки – это первое устройство в схеме очистных сооружений. Они представляют...
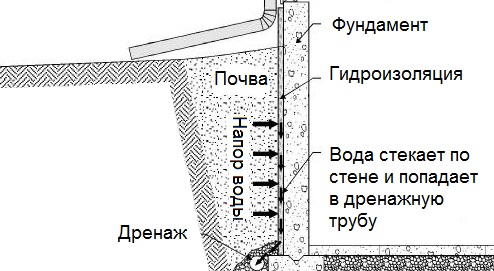
Общие условия выбора системы дренажа: Система дренажа выбирается в зависимости от характера защищаемого...

Состав сооружений: решетки и песколовки: Решетки – это первое устройство в схеме очистных сооружений. Они представляют...

Общие условия выбора системы дренажа: Система дренажа выбирается в зависимости от характера защищаемого...
Топ:
Методика измерений сопротивления растеканию тока анодного заземления: Анодный заземлитель (анод) – проводник, погруженный в электролитическую среду (грунт, раствор электролита) и подключенный к положительному...
Установка замедленного коксования: Чем выше температура и ниже давление, тем место разрыва углеродной цепи всё больше смещается к её концу и значительно возрастает...
Характеристика АТП и сварочно-жестяницкого участка: Транспорт в настоящее время является одной из важнейших отраслей народного...
Интересное:
Берегоукрепление оползневых склонов: На прибрежных склонах основной причиной развития оползневых процессов является подмыв водами рек естественных склонов...
Влияние предпринимательской среды на эффективное функционирование предприятия: Предпринимательская среда – это совокупность внешних и внутренних факторов, оказывающих влияние на функционирование фирмы...
Финансовый рынок и его значение в управлении денежными потоками на современном этапе: любому предприятию для расширения производства и увеличения прибыли нужны...
Дисциплины:
|
из
5.00
|
Заказать работу |
|
|
|
|
Determination of relative density.
Laboratory work # 13
Assessment of quality of liquors
Objective: To determine the quality of liquor-vodka beverages on physicochemical parameters.
Theoretically part
Emulsion liqueurs - it's milk, butter, egg and other liquors with fillers, flavorings, and without them. are a relatively new group of beverages, which are in high demand because of an unusual and pleasant mild flavor, low strength and attractive appearance. Currently, the Kazakh consumer market in a wide assortment of cream liqueurs mostly foreign manufacturers. Production of cream liqueur is a complex multistage process with the use of expensive equipment, resulting in increased production costs. In terms of rigidity of competition, expensive technology reduces the interest of domestic producers in the production of such beverages. Liqueurs are classified by the nature of the filler. The main types of the latter are herbs, fruits, nuts, coffee and eggs and cream.
Necessary equipment and materials: Natural samples of liquor-vodka beverages. Standards of liquor-vodka beverages. Catalogues "Liquor-vodka beverages."
Pipette with a capacity of 10, 5, 1, 20, 25 cm3. Test tubes ПП 20- КШ 10/19. Liquid Glass Thermometers. Glasses for tasting. Measuring flasks with graduated neck guilt. Flasks 2-500-2; 2-250-2; П-500, П-1000, 2-200-2, КН-100, КН-200, КН-250. Stopwatch. Areometer for spirit of АСП-1 or АСП-2. Cylinders 1-50, 1-100, 1-500. Stove. Glass tubes. Drop catcher. Bath water. Glass rods. Scales. Desiccator. Dropper. Funnel. Burettes capacity 25 cm3. Porcelain plate. Potentiometer. Laboratory refractometer. Glass refrigerator.
Hydrochloric acid, solution of sodium hydroxide (concentration 1 and 0.1 mol/dm3), a solution of phenolphthalein (1%), distilled water, a solution of Fehling I and II; solution of blue bromothymol (0.05 %).
Work progress
Determining the completeness of filling. The product of the bottle caution pricks on the wall is poured into a clean, dry flask or a flask with graduated neck. The volume of the product drain test after discharge and holding a bottle for 0.5 min over a funnel placed in a flask.
Underfilling quantify the introduction of a measuring flask to the mark of an additional quantity of product pipette with a scale division of 0.05 cm3.
Overflow quantify the removal of excess product from the measuring flask to the mark with a pipette with a scale division of 0.05 cm3.
Determine the completeness of filling at a temperature of 20 ± 0,5°C. At a different temperature into account a correction for temperature ГОСТ Р 51135).
Determination of the fortress hydrometer. The method is based on the measurement of the volume fraction ethyl alcohol hydrometer in the distillyate obtained after a preliminary distillation of alcohol from beverage.
Liquor-vodka beverages are poured up to the mark in a volumetric flask with a capacity of 250-500 cm3 at 20°C, carried of the volumetric flask to the distillation flask 500-1000 cm3. The remains of the product wash off with distilled water from volumetric flask in the distillation flask in such a way that the dimension of distilled water did not exceed 60-100 cm3.
|
|
The distillation flask connected to a vertical or direct-flow refrigerators by glass tube or drop catcher and the contents is distilled.
Volumetric flask which measure the test product is a receiving flask for the. In the flask prior before the distillation, pour 10-15 cm3 of distilled water and immersed the flask in a bath with cold water. The narrow end of a glass tube of refrigerator is placed in water at receiving flask. After filling in the receiving flask to about half of its volume the distillation water continues without the gate.
After receiving flask filled with a 3/4 of volume, the distillation is stopped. The flask with the distillate topped up with distilled water just below the label and stand 20-30 min at 20 ° C in a water bath.
Then contents of receiving flask adjusted to the mark with distillate water. Vigorously mixed, poured into a dry glass cylinder for areometer to determine the amount of alcohol stake hydrometer for alcohol or metal alcoholometer.
Determination of mass concentration of total extract by refractometric method. The method is based on definition of mass concentration of dry matter on a scale refractometer at 20° C.
The contents of flask remaining after the distillation of alcohol, washed with distilled water without loss to the flask with a capacity of 200-500 cm3 and at temperature of 20° C lead with distillate water to the mark. The solution in the flask carefully stir and bring a drop of test solution at the bottom prism of refractometer with fused glass stick. The upper part of prism is lowered, tightly applied to the lower fixed part of prism and note readouts on scale of refractometer.
If the refractometer readouts noted at a temperature different from 20°C, it is necessary to introduce an amendment (ГОСТ 51135).
The discrepancy between two parallel determinations should not exceed 0.1%.
Determination of mass concentration of sugar by method of direct titration. The method is based on titration defined quantity of Fehling solution (oxidizer) of known concentration of a solution containing sugar, until the recovery of copper oxide (2 +) in the copper protoxide (1 +).
The method used for quality control of products, and also in event of disagreement in the assessment of quality. Drawbacks of method ± 1,8%.
The test product was diluted with distilled water (see Table. 3, ГОСТ 51135).
Measure with a pipette a 25 cm3 of test product diluted in a volumetric flask with a capacity of 100 cm3. To a solution flow 25 cm3 of distilled water, and 3cm3 of hydrochloric acid (density 1.90 g/cm3). The contents of flask stirred and heated on a water bath for 5 min at 67-70°C with frequent stirring.
Then solution rapidly cooled and neutralized with sodium hydroxide of concentration 1mol/dm3 in presence of phenolphthalein. After inversion and neutralization the contents of the flask at 20°C lead with distillated water to mark and mix thoroughly.
In a conical flask with a capacity of 150-200 cm3 measure off 10 cm3 of solution Fehling I and II, and heated to boiling, add 2-3 drops of methylene blue, and boiling without stopping, from a burette with a levigated crane gently and slowly poured into the flask invert sugar antill disappearance of blue color. Then add 2-5 drops of methylene blue solution (1 percent) to mixture and boiling without stopping, continued to flow of invert sugar solution dropwise until the color of boiling mixture goes into the red or orange.
|
|
The duration of liquid boiling in flask during the titration should not exceed 3 minutes, then from the mark as consumed in the titration of the sugar solution, which is considered indicative. Then re-titration is carried out, but to a mixture of Fehling solutions I and II were added to the flask heated by 0.5-1.0 cm3 of invert sugar less than expend to the first titration.
The mixture in a flask boiled for 2 minutes, continuing boiling, add 3-5 drops of methylene blue solution. Then flow 2-3 drops of invert sugars from a burette, giving a mixture after each addition simmer 2-3 seconds until blue colouring disappears in a flask and the mixture does not take a red or orange colour. After that note the quantity of solution expended for titration.
Mass concentration of sugar (Xsugar) in recalculation on sucrose in grams per 100 cm3 of test solution is calculated by the formula
Хsugar= K 100 n ·0,95,
V
where K - correction coefficient to the titre of Fehling solution I and II;
100 - volume of analyzed product, after inversion, cm3;
n - the coefficient of dilution of analyzed beverage;
0.95 – recalculation coefficient of invert sugar in total sugar;
V - total volume of test solution expended to titration cm3.
The final result of test should be the arithmetical average of the results two parallel definitions which allowed differences between them should not exceed 5%.
Determination of mass concentration of acid. Mass acid concentration of liquor- vodka products determined by acidimetric method and method of electrometrically titration.
The acidimetric titration method is based on titration of test product with sodium hydroxide solution until a neutral reaction determining with indicator.
Measure with a pipette 10 cm3 of test product is transferred into a conical flask with a capacity of 100-250 cm3, added boiled distilled water: for the light-coloured products - 25-30cm3, and for dark-coloured - 100 cm3.
The contents of flask stirred with fused glass stick and titrated with sodium hydroxide concentration 0.1 mol/dm3 in the presence of an indicator, bromothymol blue or phenolphthalein. The end of titration determine by appearance of a light green colour in the drop test on a porcelain plate with using of bromothymol blue and pink - by using phenolphthalein.
The mass concentration of acid (C) in grams of citric acid in 100 cm3 (g/cm3) of researching product, calculated by the formula
С= VK ·100 · 0,0064 = 0,064VK,
where V - volume of solution sodium hydroxide expended in the titration, cm3;
0.0064 - mass of citric acid conformed to 1 cm3 of sodium hydroxide concentration of 0.1 mol/dm3;
10 - volume of test product, cm3;
K - correction coefficient to the titre of solution sodium hydroxide concentration of 0.1 mol/dm3.
The final result of test should be the arithmetical average of the results two parallel definitions which allowed differences between them should not exceed 5%.
Method of electrometric titration is based on titration of test product with sodium hydroxide solution until a neutral reaction determining with potentiometer.
In a glass with a capacity of 100cm3 measure 10 cm3 of test products, added 50 cm3 of distilled water, stirred and heated to boiling and cooled to room temperature. The solution titrated with sodium hydroxide concentration of 0.1 mol/dm3 in small portions, and then drop by drop. After each addition of solution the liquid in flask stirred and observed the readouts of potentiometer. The titration finished at pH 7.0.
Mass concentration of acid is calculated at indicated titration by quantity of expended sodium hydroxide solution, subsequent recalculation of citric acid in grams per 100 cm3.
The results of assessment physical-chemical parameters of quality liquor-vodka beverages draw into a table 12.
|
|
|
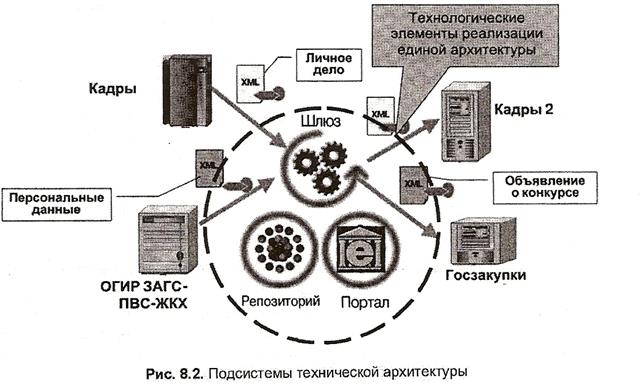
Архитектура электронного правительства: Единая архитектура – это методологический подход при создании системы управления государства, который строится...

Кормораздатчик мобильный электрифицированный: схема и процесс работы устройства...

Организация стока поверхностных вод: Наибольшее количество влаги на земном шаре испаряется с поверхности морей и океанов (88‰)...

Своеобразие русской архитектуры: Основной материал – дерево – быстрота постройки, но недолговечность и необходимость деления...
© cyberpedia.su 2017-2024 - Не является автором материалов. Исключительное право сохранено за автором текста.
Если вы не хотите, чтобы данный материал был у нас на сайте, перейдите по ссылке: Нарушение авторских прав. Мы поможем в написании вашей работы!